Selection for Social Signalling Drives the Evolution of Chameleon Colour Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Freshwater Fishes
WESTERN CAPE PROVINCE state oF BIODIVERSITY 2007 TABLE OF CONTENTS Chapter 1 Introduction 2 Chapter 2 Methods 17 Chapter 3 Freshwater fishes 18 Chapter 4 Amphibians 36 Chapter 5 Reptiles 55 Chapter 6 Mammals 75 Chapter 7 Avifauna 89 Chapter 8 Flora & Vegetation 112 Chapter 9 Land and Protected Areas 139 Chapter 10 Status of River Health 159 Cover page photographs by Andrew Turner (CapeNature), Roger Bills (SAIAB) & Wicus Leeuwner. ISBN 978-0-620-39289-1 SCIENTIFIC SERVICES 2 Western Cape Province State of Biodiversity 2007 CHAPTER 1 INTRODUCTION Andrew Turner [email protected] 1 “We live at a historic moment, a time in which the world’s biological diversity is being rapidly destroyed. The present geological period has more species than any other, yet the current rate of extinction of species is greater now than at any time in the past. Ecosystems and communities are being degraded and destroyed, and species are being driven to extinction. The species that persist are losing genetic variation as the number of individuals in populations shrinks, unique populations and subspecies are destroyed, and remaining populations become increasingly isolated from one another. The cause of this loss of biological diversity at all levels is the range of human activity that alters and destroys natural habitats to suit human needs.” (Primack, 2002). CapeNature launched its State of Biodiversity Programme (SoBP) to assess and monitor the state of biodiversity in the Western Cape in 1999. This programme delivered its first report in 2002 and these reports are updated every five years. The current report (2007) reports on the changes to the state of vertebrate biodiversity and land under conservation usage. -

Bradypodion Caeruleogula, Eshowe Dwarf Chameleon
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T172551A110305774 Scope: Global Language: English Bradypodion caeruleogula, Eshowe Dwarf Chameleon Assessment by: Tolley, K. View on www.iucnredlist.org Citation: Tolley, K. 2017. Bradypodion caeruleogula. The IUCN Red List of Threatened Species 2017: e.T172551A110305774. http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T172551A110305774.en Copyright: © 2017 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Chordata Reptilia Squamata Chamaeleonidae Taxon Name: Bradypodion caeruleogula Raw & Brothers, 2008 Common Name(s): • English: Eshowe Dwarf Chameleon, Dhlinza Dwarf Chameleon, uMlalazi Dwarf Chameleon Taxonomic Notes: Recently described from Dlinza Forest (Raw and Brothers 2008). -

Reptilian Eyes and Orbital Structures
REPTILIAN EYES AND ORBITAL STRUCTURES Jeanette Wyneken, PhD Florida Atlantic University, Dept. of Biological Sciences, 777 Glades Road, Boca Raton, FL 33431 USA ABSTRACT The anatomy of the reptilian eye is similar across species, generally, but the eyes of various taxa differ in details. The eyeball is formed of layers and has three chambers. Pupil shape differs among reptilian taxa with behavior. The retina’s photosensitive cells (rods and cones or all- cones) transmit signals to the optic nerve, which is an extension of the brain. Retinal sensitivity is increased by foveae in lizards and tuataras, while turtles and snakes have areas. The lens is soft and is shaped for accommodation by the ciliary muscles (lizards, turtles and crocodilians) or movement (snakes). All reptiles have eyelids, however some lizards have partially fused lids, while other lizards and all snakes have fused clear lids, the spectacle. Ocular glands lubricate the cornea in all species. Movements of the eyes are critical to preventing photoreceptor fatigue and loss of image recognition. INTRODUCTION TO THE REPTILIAN EYE Reptilian eyes are anatomically similar to those of other vertebrates in that the eyeball (= globe) is formed of layers, filled with fluid, and has a lens that focuses light on a retina. The eye is structured as a series of chambers. The anterior chamber is the fluid-filled space inside the eye between the iris and the cornea's innermost surface; the posterior chamber is a small space directly posterior to the iris, anterior to the lens, and bordered by the ciliary body or ciliary muscles. The anterior and posterior chambers are filled with aqueous humour. -

Kwazulu Dwarf Chameleon Bradypodion Melanocephalum Habitat Assessment for the Proposed Rohill Development, Durban, Kwazulu-Natal
KwaZulu Dwarf Chameleon Bradypodion melanocephalum habitat assessment for the proposed Rohill Development, Durban, KwaZulu-Natal Performed for GCS Water and Environment (Pty) Ltd Final Report Prepared by Harvey Ecological July 2014 1 1. INTRODUCTION Harvey Ecological was commissioned to conduct an assessment of the property for the proposed Rohill development in terms of its suitability for supporting the KwaZulu Dwarf Chameleon Bradypodion melanocephalum (hereafter KDC, also known as Black-headed Dwarf Chameleon). The KDC is globally restricted to south-eastern KwaZulu-Natal, extending slightly into the extreme northern Eastern Cape, and has a highly fragmented distribution. A number of populations are morphologically distinct and require further study to assess their relationships (Da Silva & Tolley 2013, Tolley 2014). The coastal population appears to be centred on the eThekwini municipal area, and apparently separated from the interior population (Armstrong 2009, Tolley 2014). The KDC has recently been evaluated as qualifying for the category Vulnerable, as defined by the IUCN Redlist criteria (Bates et al. 2014), given its small and declining extent of occurrence, and the highly fragmented nature and declining extent and quality of the habitat available for the species. It is also considered inadequately represented in formally protected areas (Armstrong 2001). The following terms of reference were agreed on for this assessment: 1. Undertake a site visit to identify potential KwaZulu Dwarf Chameleon Bradypodion melanocephalum habitats on site, based on the latest current understanding of their habitat requirements. 2. Provide a statement regarding the expected likelihood of occurrence of this species for each of the habitats identified. 2. METHODS 2.1 Study Site The site is ca. -

The Diversity and Adaptive Evolution of Visual Photopigments in Reptiles Frontiers in Ecology and Evolution, 7: 352
http://www.diva-portal.org This is the published version of a paper published in Frontiers in Ecology and Evolution. Citation for the original published paper (version of record): Katti, C., Stacey-Solis, M., Anahí Coronel-Rojas, N., Davies, W I. (2019) The Diversity and Adaptive Evolution of Visual Photopigments in Reptiles Frontiers in Ecology and Evolution, 7: 352 https://doi.org/10.3389/fevo.2019.00352 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-164181 REVIEW published: 19 September 2019 doi: 10.3389/fevo.2019.00352 The Diversity and Adaptive Evolution of Visual Photopigments in Reptiles Christiana Katti 1*, Micaela Stacey-Solis 1, Nicole Anahí Coronel-Rojas 1 and Wayne Iwan Lee Davies 2,3,4,5,6 1 Escuela de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador, Quito, Ecuador, 2 Center for Molecular Medicine, Umeå University, Umeå, Sweden, 3 Oceans Graduate School, University of Western Australia, Crawley, WA, Australia, 4 Oceans Institute, University of Western Australia, Crawley, WA, Australia, 5 School of Biological Sciences, University of Western Australia, Perth, WA, Australia, 6 Center for Ophthalmology and Visual Science, Lions Eye Institute, University of Western Australia, Perth, WA, Australia Reptiles are a highly diverse class that consists of snakes, geckos, iguanid lizards, and chameleons among others. Given their unique phylogenetic position in relation to both birds and mammals, reptiles are interesting animal models with which to decipher the evolution of vertebrate photopigments (opsin protein plus a light-sensitive retinal chromophore) and their contribution to vision. -

Universityof Cape Town
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive licensety ofgranted Cape to UCTTown by the author. si Univer DEMOGRAPHIC AND GENETIC VARIABILITY IN CAPE DWARF CHAMELEONS, Bradypodion pumilum, WITHIN A FRAGMENTED, URBAN HABITAT MASTER OF SCIENCE CANDIDATE: Eric Michael Katz [email protected] SUPERVISORS: Dr.y K. of A. Tolley Cape Town South African tNational Biodiversity Institute si Dr. J. M. Bishop Department of Zoology, University of Cape Town Univer & Dr. R. Altwegg South African National Biodiversity Institute Thesis presented for the degree of Master of Science Within the Department of Zoology University of Cape Town, South Africa June, 2012 (2nd Submission) PLAGIARISM DECLARATION 1. I know that plagiarism is wrong. Plagiarism is to use another’s work and pretend that it is one’s own. 2. Each contribution to, and quotation in, this thesis from the work(s) of other people has been attributed, and has been cited and referenced. 3. This thesis is my own work. 4. I have not allowed, and will not allow, anyone to copy my work with the intention of passing it off as his or her own work. 5. I acknowledge that copying someone else’s assignment or essay, or part of it, is wrong, and declare that this is my own work. _________________________ Signature Removedty of Cape Town Eric M. -

Operator's Manual Chameleon Ultra™ and Chameleon Vision™ Diode
Operator’s Manual Chameleon Ultra™ and Chameleon Vision™ Diode-Pumped Lasers 5100 Patrick Henry Drive Santa Clara, CA 95054 Chameleon Ultra & Chameleon Vision Operator’s Manual This document is copyrighted with all rights reserved. Under the copyright laws, this document may not be copied in whole or in part or reproduced in any other media without the express written permission of Coherent, Inc. Permitted copies must carry the same proprietary and copyright notices as were affixed to the original. This exception does not allow copies to be made for others, whether or not sold, but all the material purchased may be sold, given or loaned to another person. Under the law, copying includes translation into another language. Coherent, the Coherent Logo, Chameleon Ultra, Chameleon Vision, Verdi, PowerTrack, FAP-I and FieldMax are registered trademarks of Coherent, Inc. Every effort has been made to ensure that the data given in this document is accurate. The information, figures, tables, specifications, part numbers and schematics contained herein are subject to change without notice. Coherent makes no warranty or representation, either expressed or implied, with respect to this document. In no event will Coherent be liable for any direct, indirect, special, incidental or consequential damages resulting from any defects in its documentation. Technical Support In the United States: Should you experience any difficulties with your laser or need any technical information, visit our web site www.Coherent.com. Additional support can be obtained by contacting our Technical Support Hotline at 1-800-367-7890 (1-408-764-4557 outside the U.S.) or E-mail ([email protected]). -

From the Aberdare Mountains in the Central Highlands of Kenya
Zootaxa 3391: 1–22 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2012 · Magnolia Press ISSN 1175-5334 (online edition) A new species of chameleon (Squamata: Chamaeleonidae) from the Aberdare Mountains in the central highlands of Kenya JAN STIPALA1,4, NICOLA LUTZMANN2, PATRICK K. MALONZA3, PAUL WILKINSON1, BRENDAN GODLEY1, JOASH NYAMACHE3 & MATTHEW R. EVANS1 1School of Biosciences, University of Exeter, Tremough Campus, Penryn, Cornwall, TR10 9EZ, UK. E-mail: [email protected], [email protected] , [email protected], [email protected] 2Seitzstrasse 19, 69120 Heidelberg, Germany. Email: [email protected] 3Herpetology section, National Museums of Kenya, Museum Hill, Nairobi, Kenya. E-mail: [email protected], [email protected] 4Corresponding author. E-mail: [email protected] Abstract We describe a new species of chameleon, Trioceros kinangopensis sp. nov., from Kinangop Peak in the Aberdare moun- tains, central highlands of Kenya. The proposed new species is morphologically and genetically distinct from other mem- ber of the bitaeniatus-group. It is morphologically most similar to T. schubotzi but differs in the lack of sexual size dimorphism, smaller-sized females, smoother, less angular canthus rostrales, smaller scales on the temporal region and a bright orange gular crest in males. Mitochondrial DNA indicates that the proposed new taxon is a distinct lineage that is closely related to T. nyirit and T. schubotzi. The distribution of T. kinangopensis sp. nov. appears to be restricted to the afroalpine zone in vicintiy of Kinangop Peak and fires may pose a serious threat to the long-term survival of this species. -
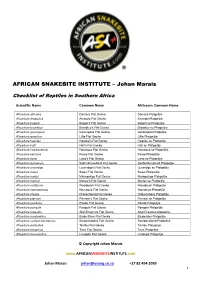
Johan Marais
AFRICAN SNAKEBITE INSTITUTE – Johan Marais Checklist of Reptiles in Southern Africa Scientific Name Common Name Afrikaans Common Name Afroedura africana Damara Flat Gecko Damara Platgeitjie Afroedura amatolica Amatola Flat Gecko Amatola Platgeitjie Afroedura bogerti Bogert's Flat Gecko Bogert se Platgeitjie Afroedura broadleyi Broadley’s Flat Gecko Broadley se Platgeitjie Afroedura gorongosa Gorongosa Flat Gecko Gorongosa Platgeitjie Afroedura granitica Lillie Flat Gecko Lillie Platgeitjie Afroedura haackei Haacke's Flat Gecko Haacke se Platgeitjie Afroedura halli Hall's Flat Gecko Hall se Platgeitjie Afroedura hawequensis Hawequa Flat Gecko Hawequa se Platgeitjie Afroedura karroica Karoo Flat Gecko Karoo Platgeitjie Afroedura langi Lang's Flat Gecko Lang se Platgeitjie Afroedura leoloensis Sekhukhuneland Flat Gecko Sekhukhuneland Platgeitjie Afroedura loveridgei Loveridge's Flat Gecko Loveridge se Platgeitjie Afroedura major Swazi Flat Gecko Swazi Platgeitjie Afroedura maripi Mariepskop Flat Gecko Mariepskop Platgeitjie Afroedura marleyi Marley's Flat Gecko Marley se Platgeitjie Afroedura multiporis Woodbush Flat Gecko Woodbush Platgeijtie Afroedura namaquensis Namaqua Flat Gecko Namakwa Platgeitjie Afroedura nivaria Drakensberg Flat Gecko Drakensberg Platgeitjie Afroedura pienaari Pienaar’s Flat Gecko Pienaar se Platgeitjie Afroedura pondolia Pondo Flat Gecko Pondo Platgeitjie Afroedura pongola Pongola Flat Gecko Pongola Platgeitjie Afroedura rupestris Abel Erasmus Flat Gecko Abel Erasmus platgeitjie Afroedura rondavelica Blyde River -

Integrated Reserve Management Plan
INTEGRATED RESERVE MANAGEMENT PLAN ZANDVLEI ESTUARY NATURE RESERVE June 2011 AUTHORIZATION PAGE This Integrated Management Plan for the Zandvlei Estuary Nature Reserve was drafted by the Area Manager and recommended by the Reserve Planning Team, a multi-disciplinary team consisting of: Reserve Planning Team: Branch Manager, Regional Manager, Area Manager, Biophysical Specialist, Biodiversity Coordinator, Sustainable Resource Specialist and Monitoring and Evaluation Coordinator _____________________________________ Casandra Sheasby – Area Manager Biodiversity Management Branch Environmental Resource Management Department City of Cape Town Name and Title Signature and Date Mr Jacob Hugo Executive Director: Economic, Environment and Spatial Planning Directorate Approved by: Name and Title Signature and Date Mr Anton Bredell Minister of Local Government, Environmental Affairs and Development Planning DOCUMENTED Integrated Reserve Management Plan This management plan replaces any previous versions Document Version: Prepared by: Adele Pretorius Version.02: June 2011 Responsibility: Reserve Management plans Document reference: Approved by: Mr Anton Bredell (DEA&DP reference to be Position: Minister of Local Government, Environmental inserted) Affairs and Development Planning Date of approval: Date of implementation : Immediate Distribution: Distribute as Reserve Management Plan to relevant staff in the City of Cape Town for immediate implementation. Date for Revision: June 2016 INTEGRATED RESERVE MANAGEMENT PLAN Compiled by Dalton Gibbs, Vaughn Thompson and Cassandra Sheasby Biodiversity Management Branch Environmental Resource Management Department City of Cape Town ZANDVLEI ESTUARY NATURE RESERVE June 2011 ISBN NUMBER TABLE OF CONTENTS PART SECTIONS AND SUBSECTIONS PAGE NUMBER NUMBER List of maps ii List of figures ii List of tables ii List of appendices iii List of abbreviations used iv Part 1 1. -

Phd Thesis Jennifer C. Jackson 16.10.07 For
REPRODUCTION IN DWARF CHAMELEONS (BRADYPODION) WITH PARTICULAR REFERENCE TO B. PUMILUM OCCURRING IN FIRE-PRONE FYNBOS HABITAT JENNIFER C. JACKSON Dissertation presented for the degree of Doctor of Philosophy (Zoology) at the University of Stellenbosch Supervisor: Prof. P le F. N. Mouton Co-supervisor: Dr. A. F. Flemming December 2007 Stellenbosch University http://scholar.sun.ac.za DECLARATION I, the undersigned, hereby declare that the work contained in this thesis is my own original work and that I have not previously in its entirety or in part been submitted it at any university for a degree. ………………………………. ……………… Signature Date Copyright © 2007 Stellenbosch University All rights reserved II Stellenbosch University http://scholar.sun.ac.za ABSTRACT South Africa, Lesotho and Swaziland are home to an endemic group of dwarf chameleons (Bradypodion). They are small, viviparous, insectivorous, arboreal lizards, found in a variety of vegetation types and climatic conditions. Previous work on Bradypodion pumilum suggests prolonged breeding and high fecundity which is very unusual for a viviparous lizard inhabiting a Mediterranean environment. It has been suggested that the alleged prolonged reproduction observed in B. pumilum may be a reproductive adaptation to life in a fire-prone habitat. In addition, Chamaesaura anguina a viviparous, arboreal grass lizard also occurs in the fire-frequent fynbos and exhibits an aseasonal female reproductive cycle with high clutch sizes; highly unusual for the Cordylidae. With the observation of two species both inhabiting a fire-driven environment and exhibiting aseasonal reproductive cycles with high fecundity, it was thought that this unpredictable environment may shape the reproductive strategies of animals inhabiting it. -

Havens of Biodiversity, and Places That Allow People to Connect with Natural Habitats and Ecosystems, Will Become Increasingly More Valuable for Future Generations
Supplement to Veld & Flora, Vol. 93(4) December 2007 1 booklet3_FINAL_for print.indd 1 2007/11/02 10:50:33 AM FOREWORD The Botanical Society of South Africa (BotSoc) has been a partner and supporter of the South African National Biodiversity Institute (SANBI) and its forerunners for over 90 years. This supplement to Veld & Flora focuses on other “biodiversity” (birds, mammals, insects, etc.) rather than just our core interest, which is “plant diversity”. It is an example of BotSoc embracing the change which Dr Bruce McKenzie has come about since SANBI replaced its predecessor Executive Director, BotSoc the National Botanical Institute (NBI) and also supports one of the principles contained in BotSoc’s Centenary Charter (see Veld & Flora, March 2006) which outlines our commitment to supporting SANBI and its mandate. In this regard the BotSoc warmly welcomes the first CEO of SANBI, Dr Tanya Abrahamse, and looks forward to working with her and her team in tackling new challenges, some of which she has spelt out in her foreword to the supplement. Dr Bruce McKenzie EXECUTIVE DIRECTOR, BotSoc CONTENTS 2 Animals form an integral part of South Africa’s National Botanical Gardens 3 Free State NBG, Bloemfontein 4 Harold Porter NBG, Betty’s Bay 6 Karoo Desert NBG, Worcester 7 Kirstenbosch NBG, Cape Town KwaZulu-Natal NBG, Pietermaritzburg Compiled by: 11 Christopher K. Willis & 13 Lowveld NBG, Nelspruit Augustine T. Morkel 16 Nieuwoudtville NBG Published by: The Botanical Society of South Africa 18 Pretoria NBG and the South African National 21