March 29, 2021 from Dr. P. Dileep Assistant Director, J J Murphy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
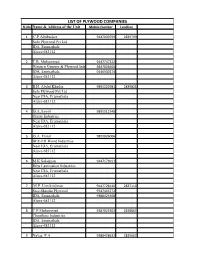
LIST of PLYWOOD COMPANIES Sl.No Name & Address of the Unit Mobile Number Landline
LIST OF PLYWOOD COMPANIES Sl.No Name & Address of the Unit Mobile Number Landline 1 C.P.Abubacker 9447039799 2839799 Solo Plywood Pvt Ltd IDA, Erumathala Aluva-683112 2 T.B. Muhammed 9447747222 Western Veneers & Plywood Industries 9847804668 IDA, Erumathala 9446939274 Aluva-683112 3 B.H. Abdul Khader 9895222981 2839821 Safa Plywood Pvt Ltd Near IDA, Erumathala Aluva-683112 4 B.A.Ayoob 9895512348 Orient Industries Near IDA, Erumathala Aluva-683112 5 B.A. Faisal 9809026006 BEE-EH Wood Industries Near IDA, Erumathala Aluva-683112 6 M.K.Sulaiman 9447171011 Ribu Lamination Industries Near IDA, Erumathala Aluva-683112 7 M.P. Unnikrishnan 9447728140 2837110 Sree Shastha Plywood 9747403772 IDA, Erumathala 9388423368 Aluva-683112 8 C.P.Muhammed 9387025302 2838865 Chenthara Industries IDA, Erumathala Aluva-683112 9 Navas. P.A 9388428632 2839662 Global Conductors IDA, Erumathala Aluva-683112 10 P.M.R.Musthafa 9447182934 Peeyemmar Plywood Industries IDA, Erumathala Aluva-683112 11 C.P.Muhammed 9447604923 2839315 Skyblue Plywoods IDA, Erumathala Aluva-683112 12 Muhammed Shiyas 9895868494 2839687 Usmania Wood Decors 9388881019 Near IDA, Erumathala Aluva-683105 13 M.A.Ashraf 3234321 Feroke Boards Behind ISRO Nalam Mile, Erumathala Aluva-683112 14 T.A. Subair 9388636080 Modem Industry Keenpuram S.Vazhakkulam 15 P.J.Abubakker 9846308880 2677044 Tens Rubber Company Pvt Ltd IDA, Keenpuram S.Vazhakkulam-683105 16 V.M.Anwar 9388604554 Eden Plywood S.Eripuram S.Vazhakkulam-683105 17 T.I.Jaffer 9388961884 2594216 Elavumkudy Veneers 9846128861 591607 Ponjassery.P.O -

ERNAKULAM DISTRICT GENERAL CATEGORY Sl
NATIONAL MEANS CUM MERIT SCHOLARSHIP EXAMINATION (NMMSE)-2019 (FINAL LIST OF ELIGIBLE CANDIDATES) ERNAKULAM DISTRICT GENERAL CATEGORY Sl. Caste ROLL NO Applicant Name School_Name No Category 1 42192300339 NIMISHA SHINOB General St. Paul`s H S Veliyanad , veliyanad 2 42192290136 ANEETTA SIBY General H.S.S Valayanchirangara , Valayanchirangara 3 42192300164 DEVAPRIYA SANTHOSH General St. Augustine`s Girls H S S Muvattupuzha , Muvattupuzha 4 42192220018 ABHIJITH P V General Govt. H.S.S And V.H.S.S. Kadamakudy , Kadamakudy 5 42192240151 HANNA JUSTIN General St. Joseph`s H. S. for Girls Varapuzha , EDAMPADAM 6 42192300109 ABRON SAJI PACKAL General Sacred Heart H S Ayavana , AYAVANA 7 42192290098 ARUNIMA MADHU General Govt. H S for Girls Perumbavoor , PERUMBAVOOR 8 42192220119NEHA V General St. Thomas Girls H.S. Perumanoor , Pandit Karuppan Road 9 42192250096 NANDANA REMESH General Govt. H. S. S. Kadayirippu , Kolenchery 10 42192300288 ANIT JOB General M K M H S Piravom , Piravom St. Joseph`s H. S. for Girls Varapuzha , EDAMPADAM, 11 42192260169 SONA SHIBU General VARAPUZHA P.O. 12 42192290134 ALEENA BIJU General H.S.S Valayanchirangara , Valayanchirangara 13 42192290082 ABHINAND PRAKASH General M G M H S S Kuruppampady , Kuruppampady 14 42192290112 ANNA MARIA ELDHOSE General S N H S S Okkal , okkal 15 42192280143 MALAVIKA RAJAN General St. Augustine`s Girls H S S Kothamangalam , 16 42192220097 JINSA SEBASTIAN General C.C.P.L.M.A.I.H.S. Perumanoor , M G ROAD 17 42192290005 ANNA ROSE MATHEW General St. Joseph`s H S S Paingottoor , Paingottoor 18 42192270165 ADITH ANILKUMAR General N. S. S. H. -

Ernakulam District, Kerala State
TECHNICAL REPORTS: SERIES ‘D’ CONSERVE WATER – SAVE LIFE भारत सरकार GOVERNMENT OF INDIA जल संसाधन मंत्रालय MINISTRY OF WATER RESOURCES कᴂ द्रीय भूजल बो셍 ड CENTRAL GROUND WATER BOARD केरल क्षेत्र KERALA REGION भूजल सूचना पुस्तिका, एर्ााकु लम स्ज쥍ला, केरल रा煍य GROUND WATER INFORMATION BOOKLET OF ERNAKULAM DISTRICT, KERALA STATE तत셁वनंतपुरम Thiruvananthapuram December 2013 GOVERNMENT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD GROUND WATER INFORMATION BOOKLET OF ERNAKULAM DISTRICT, KERALA 饍वारा By टी. एस अनीता �याम वैज्ञातनक ग T.S.Anitha Shyam Scientist C KERALA REGION BHUJAL BHAVAN KEDARAM, KESAVADASPURAM NH-IV, FARIDABAD THIRUVANANTHAPURAM – 695 004 HARYANA- 121 001 TEL: 0471-2442175 TEL: 0129-12419075 FAX: 0471-2442191 FAX: 0129-2142524 GROUND WATER INFORMATION BOOKLET OF ERNAKULAM DISTRICT, KERALA STATE TABLE OF CONTENTS DISTRICT AT A GLANCE 1.0 INTRODUCTION ..................................................................................................... 1 2.0 RAINFALL AND CLIMATE ................................................................................... 4 3.0 GEOMORPHOLOGY AND SOIL ............................................................................ 5 4.0 GROUND WATER SCENARIO .............................................................................. 6 5.0 GROUND WATER DEVELOPMENT AND MANAGEMENT .......................... 13 6.0 GROUND WATER RELATED ISSUES AND PROBLEMS ................................ 13 7.0 AWARENESS AND TRAINING ACTIVITY ...................................................... -

Department of Industries & Commerce District
Industrial Potential Survey of Ernakulam District GOVERNMENT OF KERALA DEPARTMENT OF INDUSTRIES & COMMERCE DISTRICT INDUSTRIES CENTRE ERNAKULAM POTENTIAL SURVEY REPORT FOR MSME SECTOR Prepared and Published by DISTRICT INDUSTRIES CENTRE KUNNUMPURAM CIVIL STATION ROAD,KAKKANAD,ERNAKULAM PH: 0484-2421432,2421461,FAX – 0484 2421461 E mail- [email protected], [email protected] Web site: www.dic.kerala.gov.in 1 Prepared & Submitted by District Industries Centre,Ernakulam Industrial Potential Survey of Ernakulam District PREFACE An Industrial Potential Survey of Ernakulam District, the industrial capital of Kerala, definitely will be a reflection of the State as a whole. The report is prepared mostly on the basis of available information in different sectors. The survey report reveals the existing industrial scenario of the district and it mainly aims to unveil the potentially disused areas of the industry in Ernakulam. We hope this document will provide guidance for those who need to identify various potential sources/ sectors of industry and thereby can contribute industrial development of the district, and the state. I hereby acknowledge the services rendered by all Managers, Assistant District Industries Officers , Industries Extension Officers ,Statistical Wing and other officers of this office ,for their sincere effort and whole hearted co- operation to make this venture a success within the stipulated time. I am grateful to all the officers of other departments who contributed valuable suggestions and information to prepare this report. General Manager, District Industries Centre, Ernakulam. 2 Prepared & Submitted by District Industries Centre,Ernakulam Industrial Potential Survey of Ernakulam District INDEX Contents Page No Scope & Objectives Methodology Chapter I District at a glance 1.1 Introduction 1.2 Location and extent 1.3 District at a glance 2. -

In the High Court of Kerala at Ernakulam Present
IN THE HIGH COURT OF KERALA AT ERNAKULAM PRESENT THE HONOURABLE MR. JUSTICE A.HARIPRASAD & THE HONOURABLE MR.JUSTICE ZIYAD RAHMAN A.A. FRIDAY, THE 07TH DAY OF MAY 2021 / 17TH VAISAKHA, 1943 FAO.No.114 OF 2020 AGAINST THE ORDER IN IOP 5/2017 DATED 06-11-2020 OF SUB COURT, MUVATTUPUZHA. APPELLANT/PETITIONER IN I.O.P/PLAINTIFF IN PROPOSED SUIT: JAGADAMMA, AGED 85 YEARS, W/O.RAMAKRISHNAN NAIR, AND D/O.KALYANI AMMA, KRISHNAVIHAR @ KOCHUKOYICKAL HOUSE, KUTHUKUZHY KARA, KUTHUKUZHY P.O., KOTHAMANGALAM VILLAGE, ERNAKULAM DISTRICT, PIN 686 691. BY ADVS. SRI.P.B.KRISHNAN SRI.P.B.SUBRAMANYAN SRI.SABU GEORGE SMT.B.ANUSREE SRI.MANU VYASAN PETER RESPONDENTS/RESPONDENTS IN I.O.P/DEFENDANTS IN PROPOSED SUIT : 1 SHEELA, AGED 64 YEARS, W/O.SASEENDRANATH, GEETHANJALI HOUSE, VALAYANCHIRANGARA KARA, VALAYANCHIRANGARA P.O., RAYAMANGALAM VILLAGE, KUNNATHUNADU TALUK, PIN-683 356. FAO No.114 of 2020 2 2 ASHA, AGED 47 YEARS, W/O.SATHEESHKUMAR V.K., AJITH BUILDING, OPPOSITE OF BALAJI COMPLEX, RAILWAY COLONY ROAD, AKATHETHARA VILLAGE, KALLEKULANGARA P.O., PALAKKADU TALUK, PALAKKAD DISTRICT. NOW RESIDING AT 9B, ABAD BUILDING, NEAR GOVT. ARTS COLLEGE, MARKET ROAD, THRIPPUNITHURA P.O., PIN 682 301. R1 BY ADV. SRI.G.SREEKUMAR (CHELUR) R2 BY ADV. SRI.THOMAS M.JACOB R2 BY ADV. V.V.CHACKO THIS FIRST APPEAL FROM ORDERS HAVING COME UP FOR ADMISSION ON 31-03-2021, THE COURT ON 07-05-2021 DELIVERED THE FOLLOWING: FAO No.114 of 2020 3 "C.R" A.HARIPRASAD & ZIYAD RAHMAN A.A., JJ. ------------------------------------- F.A.O No. OF 114 of 2020 ------------------------------------- Dated this the 7th day of May, 2021 J U D G M E N T Hariprasad, J. -

Rubber Park India (P) Ltd
RUBBER PARK INDIA (P) LTD MANUAL OF INSTRUCTIONS TO THE LESSEES (Forms part of the Lease Agreement) (Revised & Updated as on 30.06.2018) 2A, Kautileeyam, Valayanchirangara Ernakulam – 683 556 Ph: 0484-2657218/2655548 Email: [email protected] Website: www.rubberparkindia.org INDEX S/N Subject Page # From the Desk of the Managing Director 1 Salient Features 2 General 3-4 Detailed Instructions 5 1.0 Socially Responsive Investment 6 2.0 Allotment of Land 7-9 3.0 Documents to be Executed 10 4.0 Dues and Deposits 11 5.0 Drainage 12 6.0 Building Rules 13 7.0 Utilities 14 – 20 (a) Water i) Industrial ii) Drinking (b) Power (c) Waste Management (d) Testing & Certification 8.0 Other facilities 21-23 (a) Single Window Clearance (b) Investment Subsidy (c) Accommodation (i) Guest Suites (ii) Hostel for Male Executives (iii) Dormitory for Men (d) Medicare (e) Meetings (i) Board Room (ii) Conference Room (f) Library (g) Association of the Industrial Units (RUPMA) (h) Shopping Mall (i) Canteen Annexures 24 - 63 Whom to contact for what? 64 Welcome to Rubber Park………….. Undoubtedly you have made the right decision in making Rubber Park your investment destination. Rubber Park India (P) Ltd is a joint venture of Rubber Board, a statutory body constituted by the Govt. of India and Kerala Industrial Infrastructure Development Corporation (KINFRA), a Statutory Corporation constituted by the Govt. of Kerala under the Kerala Industrial Infrastructure Development Act 1993, a Company incorporated under the Companies Act 1956, having its registered office at 2A, Kautileeyam, Rubber Park Campus, Valayanchirangara – 683 556 It was incorporated on 10.12.1997. -

Higher Secondary Schools - Ernakulam
HIGHER SECONDARY SCHOOLS - ERNAKULAM ASRAM H.S.S & H.S.PERUMBAVOOR BRAHMANANDODAYAM HIGIHR SECONDARY SCHOOL (HIGH SCHOOL SECTION) KALADY CARDINAL HSS ,THRIKKAKARA JUDGEMUKKU,THRIKKAKARA PO DARUL ULOOM VHSS ERNAKULAM E.M.GOVT H.S.S FORT KOCHI, VELI FR. JOSEPH MEMORIAL HSS PUTHUPPADY(2) MUVATTUPUZHA G.G.H.S.S ALUVA NEAR MUNICIPAL OFFICE, ALUVA GOVT H.S.S ELAMAKKARA, KOCHI GOVT H.S.S PAZHAMTHOTTAM PAZHAMTHOTTAM P.O. GOVT HIGHER SECONDARY SCHOOL PERUMBAVOOR P.O GOVT HIGHER SECONDRY SCHOOL CHERANALOORE GOVT HSS (HS SECTION) MANJAPRA GOVT HSS KADAYIRIPPU ( HS SECTION ) AIKKARANADU P.O GOVT HSS MOOKKANNOR GOVT VHSS(HS) NJARAKKAL GOVT VOCATIONAL HIGHER SECONDARY SCHOOL, EAST MARADY, MUVATTUPUZHA GOVT. BOYS VHSS (HIGH SCHOOL) TRIPUNITHURA GOVT. GIRLS H.S.S. PERUMBAVOOR GOVT. HIGHER SECONDARY SCHOOL POOTHRIKKA POOTHRIKKA P O GOVT. HIGHER SECONDARY SCHOOL EDATHALA.P.O.,ALUVA GOVT. HIGHER SECONDARY SCHOOL ELANKUNNAPUZHA P.O. GOVT. HIGHER SECONDARY SCHOOL(H.S. SECTION) SOUTH EZHIPURAM GOVT. MODEL HIGHER SECONDARY SCHOOL MUVATTUPUZHA GOVT. MODEL HIGHER SECONDARY SCHOOL(H.S. SECTION) CHERUVATOOR. P.O GOVT. V H S S, CHOTTANIKKARA GOVT. V.H.S.S KALAMASSERY GOVT. VHS SCHOOL NERIAMANGALAM GOVT.GHSS CHITTOR ROAD ERNAKULAM GOVT.GIRLS H.S.S CHITTOR ROAD ERNAKULAM GOVT.H S S CHATHAMATTOM MATTANCHERY. KOCHI GOVT.H.S.S. KALLIL, METHALA GOVT.H.S.S. OORAMANA, (HIGH SCHOOL) GOVT.HIGHER SECONDARY SCHOOL , EZHIKKARA MUVATTUPUZHA GOVT.HIGHER SECONDARY SCHOOL MUPPATHADOM GOVT.HIGHER SECONDARY SCHOOL , EZHIKKARA.P.O., N.PARAVUR GOVT.HIGHER SECONDARY SCHOOL, MAMMALASSERY GOVT.HIGHER SECONDARY SCHOOL,PULIYANAM.P.O MAMMALASSEY.P.O RAMAMANGALAM GOVT.HSS PUTHIYAKAVU HIGHER SECONDARY SCHOOL OF JESUS (HIGH SCHOOL), KOTHAD HIGHER SECONDARY SCHOOL, VALAYANCHIRANGARA JAMA-ATH-H.S.S KALOOR P.O. -

Accused Persons Arrested in Ernakulam Rural District from 10.11.2019To16.11.2019
Accused Persons arrested in Ernakulam Rural district from 10.11.2019to16.11.2019 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Pallithazhathu H , Cr. 1150/19, Pallithazham , 10.11.19 at Mulanthurut Eby M P SI of 1 Nithin Surendran 21-MALE Pallithazham , U/s 279 IPC, Station Bail Mulanthuruthy 10.20 Hrs hy Police Amballoor 185 Mv act Memuriyil H , Cr. 1151/19, 10.11.19 at Mulanthurut Eby M P SI of 2 Jerin Johny 18-MALE Vattukunnu , Mulanthuruthy U/s 279 IPC, Station Bail 11.05 Hrs hy Police Mulanthuruthy 185 Mv act Karavatte Cruiz Cr. 1152/19, 10.11.19 at Mulanthurut Eby M P SI of 3 Abin . Babu Joseph . E. T 21-MALE Eravelil H , Amballoor , U/s 279 IPC, Station Bail 11.55 Hrs hy Police Mulanthuruthy 185 Mv act Egeetha Nivas , 10.11.19 at Cr. 1153/19, Mulanthurut Eby M P SI of 4 Sarath. S . Nair Sivaraman Nair29-MALE Vettikulam , Mulanthuruthy Station Bail 16.55 Hrs U/s 279 IPC hy Police Mulanthuruthy Mancheriyil H , 11.11.19 at Cr. 1154/19, Mulanthurut Eby M P SI of 5 Nivin Jose Jose 25-MALE Kavumugal Station Bail Kanjiramattom 17.10 Hrs U/s 279 IPC hy Police Moothedathil H , 11.11.19 at Cr. -

Accused Persons Arrested in Eranakulam Rural District from 21.08.2016 to 27.08.2016
Accused Persons arrested in Eranakulam Rural district from 21.08.2016 to 27.08.2016 Name of the Name of Name of the Place at Date & Court at Sl. Name of the Age & Cr. No & Sec Police Arresting father of Address of Accused which Time of which No. Accused Sex of Law Station Officer, Rank Accused Arrested Arrest accused & Designation produced 1 2 3 4 5 6 7 8 9 10 11 1 Chakravelil 21.08.2016 856/16 U/s 279 Mulanthuruth Sunil Thomas,S I Baiju Babu 30/M House,Perumbilly Mulanthuruthy at 13.25 hrs ipc,185 mv act y of police Station bail 2 Thoppil 21.08.2016 857/16 U/s 279 Mulanthuruth Sunil Thomas,S I Kurian Moncy 29/M House,Amballoor Panarpalam at 14.20 hrs ipc,185 mv act y of police Station bail 3 Gokulnivas,Arayankav 21.08.2016 858/16 U/s 279 Mulanthuruth Sunil Thomas,S I Gopakumar Gopalakrishnan 25/M u Arayankavu at 18.55 hrs ipc,185 mva ct y of police Station bail 4 Koonampalathingal 21.08.2016 859/16 U/s 279 Mulanthuruth Sunil Thomas,S I Prasanth Vijayan 36/M house,Kureekkadu Injimala at 20.00 hrs ipc,185 mv act y of police Station bail 5 Kinginisseril 22.08.2016 860/16 U/s 379 Mulanthuruth Sunil Thomas,S I JFCMC,Piravo Jinu Raju 28/M House,Palce squire Injimala at 10.00 hrs ipc y of police m 6 Kinginisseril 22.08.2016 860/16 U/s 379 Mulanthuruth Sunil Thomas,S I JFCMC,Piravo Jithu Raju 24/M House,Palce squire Injimala at 10.00 hrs ipc y of police m 7 Kalayil 22.08.2016 861/16 U/s 279 Mulanthuruth Sunil Thomas,S I Ananthu Ashokan 21/M House,kanjiramattom Kanjiramattom at 18.35 hrs ipc,185 mv act y of police Station bail 8 Cheruthottil -

Kunnathunadu Taluk, Ernakulam District
Minor Mineral Quarry details: Kunnathunadu Taluk, Ernakulam District Sl.No. Code Mineral Rock type Village Locality Owner Sl.No. Code Mineral Rock type Village Locality Owner Vadayampady - Cheryan Pathrose, Poovelil H, Vadayampady 620 Granite(Building Stone) Charnockite Aikaranad South 477 Granite(Building Stone) Charnockite Irapuram Mannur Sunny, S/o Puolose,Parithery H,Irapuram PO 1 Patham Mile PO, Puthencruz 61 Kunju,Karikottil H, Nirappamala, Kolencherry 654 Laterite Aikaranad South Nirappamala Mannur -Ganapathy 2 PO 513 Laterite Irapuram Poulose, Mannath H, Mannur, Keezhillom PO temple Kinginimattom , Ayyappan, Kadambanadu House, 705 Laterite Laterite Aikaranad South 62 Kakkattupara Kinginimattom .P.O, 3 63 514 Granite(Building Stone) Charnockite Irapuram Thrikkalathur Kuriachan, Variklalil H, Thrikalathur PO Slabs & Aggregates, Kadayiruppu.P.O, 704 Granite(Building Stone) Charnockite Aikaranadu Kadayiruppu 64 515 Granite(Building Stone) Charnockite Irapuram Thrikalathur Paulose, Molekkudy H, Thrikalathur, 4 Kolrncheri, Matheu (Retired H.M), Thottappilli House, 516 Granite(Building Stone) Charnockite Irapuram Thrikkalathur Mathappan, Palappuram H, Muvattupuzha 706 Laterite Laterite Aikaranadu Vadayambadi 65 5 Vadayambadi (P.O) Thrikalathur- Thankachan M.T., S/o Kuriachan, Moothekkattu Reni Varghese, Thazhekkudy H, Perumani, 517 Granite(Building Stone) Charnockite Irapuram 584 Granite(Building Stone) Charnockite Aikaranadu North Perumani 66 Veettoor H, Thrikkalathur, Nellad PO 6 Vengola PO P.V.Kuriakose, Pulikkakudiyil H, Koozhoor, -

Ernakulam District, Kerala
कᴂद्रीय भूमि जल बो셍ड जल संसाधन, नदी विकास और गंगा संरक्षण विभाग, जल शक्ति मंत्रालय भारत सरकार Central Ground Water Board Department of Water Resources, River Development and Ganga Rejuvenation, Ministry of Jal Shakti Government of India AQUIFER MAPPING AND MANAGEMENT OF GROUND WATER RESOURCES ERNAKULAM DISTRICT, KERALA केरल क्षेत्र, वत셁िनंतपुरम Kerala Region, Thiruvananthapuram AQUIFER MAPPING AND MANAGEMENT PLAN FOR HARD ROCK TERRAINS, ERNAKULAM DISTRICT, KERALA (AAP- 2018-19) GOVERNMENT OF INDIA MINISTRY OF JAL SHAKTI DEPARTMENT OF WATER RESOURCES, RIVER DEVELOPMENT AND GANGA REJUVENATION CENTRAL GROUND WATER BOARD AQUIFER MAPPING AND MANAGEMENT PLAN FOR HARD ROCK TERRAINS OF ERNAKULAM DISTRICT, KERALA (AAP: 2018-19) Roopesh G. Krishnan Scientist – B (JHG) Kerala Region Thiruvananthapuram December,2019 AQUIFER MAPPING AND MANAGEMENT PLAN FOR HARD ROCK TERRAINS, ERNAKULAM DISTRICT, KERALA (AAP- 2018-19) FOREWARD The National Project on Aquifer Mapping (NAQUIM) is an initiative of the Ministry of Jal Shakti, Department of Water Resources, River Development & Ganga Rejuvenation, Government of India, for mapping and managing the entire aquifer systems in the country. The aquifer systems in Kerala are being mapped as part of this Programme and this report pertains to aquifer mapping of the hard rock terrains of Ernakulam district. The target scale of investigation is 1:50,000 and envisages detailed study of the aquifer systems up to 200 m depth, to ascertain their resource, water quality, sustainability, and finally evolve an aquifer management plan. The report titled “Aquifer Mapping and Management plan for hard rock terrains, Ernakulam district, Kerala” gives a complete and detailed scientific account of the various aspects of the hard rock aquifers in the area including its vertical and horizontal dimensions, flow directions, quantum and quality of the resources, of both - the shallow and deeper zones of the hard rock aquifers. -

Directorate of Higher Secondary Education
DIRECTORATE OF HIGHER SECONDARY EDUCATION ERNAKULAM DISTRICT CLUSTER VENUE & RP LIST Centre Name of Training Subjects Sub district / Educational District Code Centre Name & address of RPs Sureshkumatr T, KPMHSS, Poothotta ENGLISH Aluva PHYSICS Aluva Edl.Dist. No RP Available CHEMISTRY Aluva Edl.Dist. No RP Available GOVT HSS COMPUTER 7003 Ernakulam, Vypin EDAPALLY SCIENCE/ APPLICATION Shiju S S, KPMHSS, Poothotta HINDI Ernakulam, Mattanchery, Vypin LIZTRESA I J, ST ANTONY'S HSS KACHERIPADY, GEOGRAPHY All Sub. Dist. JAYAPRADEEP V M, GBHSS, PERUMBAVOOR, GGHSS, SULATHA A K, GHSS KADAMAKKUDY, GGHSS, ERNAKULAM, 7005 GOVT HSS FOR ENGLISH Ernakulam Sub Dist. 7005 GIRLS ERNAKULAM PHYSICS Ernakulam Sub.Dist, Tripunithura DEEPA AP, GOVT HSS ELAMAKKARA, CHEMISTRY Ernakulam Sub. Dist, Mattanchery LINET LUIS , SRV HSS, ERNAKULAM, GGHSS, ENGLISH Paravur SALI JOSE, GHSS ELAMKUNNAPUZHA, GGHSS ALUVA, 7021 NISHA K U, GVHSS NJARAKKAL, ST.MARY'S GHSS MARKET ROAD, MATHEMATICS Aluva and North Paravur ERNAKULAM , 7073 GBHSS NORTH CHEMISTRY Paravur Sub.Dist.and Vypin SEENA N THANKAPPAN, ST FRANCIS HSS ALUVA 7010 PARAVUR PHYSICS Paravur Sub.Dist.and Vypin DEEPTHI ROY, GHSS ELAMKUNNAPUZHA Ernakulam, Mattanchery, Vypin, Aluva, HISTORY Angamaly, Tripunithura, Paravur SONY C RAGHAVAN, SN MHSS MOOTHAKUNNAM, MATHEMATICS Kolenchery and Angamaly NAWAS C A, GBHSS PERUMBAVOOR, GHSS EDAPPALLY, 7003 ECONOMICS Perumbavur, Muvattupuzha, Kolenchery Dr.SHAJI PAUL, SNDPHSS, MUVATTUPUZHA, 7053 Muvattupuzha and Kothamangalam , Joby Thomas Philip, MTM HSS, Pambakuda GOVT BOYS HSS 7011 POLITICAL SCIENCE Bosemon Joseph, HSSV, Valayanchirangara P O PERUMBAVOOR Angamaly,Aluva, Kolenchery, Kallorkadu, Koothattukulam, Perumbavur JAMES, GOVT. MODEL HSS MOOVATTUPUZHA, ERNAKULAM, ST.THERESA'S ERNAKULAM, 7069 Perumbavoor sub. Dist and Kolenchery , PHYSICS Angamaly SHIJU THOMAS, MAR BASIL HSS KOTHAMANGALAM, ENGLISH Perumbavoor sub.