Structural Basis of Substrate Recognition and Translocation by Human ABCA4 ✉ Tian Xie 1,2, Zike Zhang1,2, Qi Fang1, Bowen Du1 & Xin Gong 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Targeted Sequence Capture and High-Throughput Sequencing in the Molecular Diagnosis of Ichthyosis and Other Skin Diseases
View metadata, citation and similar papers at core.ac.uk brought to you by CORE providedCA by Elsevier Scott - Publisheret al. Connector Sequence Capture in Molecular Diagnosis of Skin Diseases Immunohistochemical staining indi- 1The Wellcome Trust Center for Human cause of Olmsted syndrome. Am J Hum cated that MBTPS2 is mainly expressed Genetics, Nuffield Department of Clinical Genet 90:558–64 Medicine, University of Oxford, Oxford, UK; Mevorah B, Goldberg I, Sprecher E et al. (2005) in the upper granular layer in normal 2 Centre for Cutaneous Research, The Blizard Olmsted syndrome: mutilating palmo- skin, as previously shown (Aten et al., Institute, Barts & The London School of plantar keratoderma with periorificial 2010); however, in OS skin, MBTPS2 Medicine and Dentistry, Queen Mary keratotic plaques. J Am Acad Dermatol 53: University of London, London, UK; S266–72 was expressed throughout the epi- 3 dermis (Figure 2c). There was no Department of Dermatology, Jundishapur Naiki M, Mizuno S, Yamada K et al. (2012) University of Medical Sciences, Ahvaz, Iran; MBTPS2 mutation causes BRESEK/BRESHECK 4 apparent difference in MBTPS2 locali- Genetic Department, Kerman University of syndrome. Am J Med Genet A 158A:97–102 zation in the skin of a KFSD patient with 5 Medical Sciences, Kerman, Iran and Darwin Oeffner F, Fischer G, Happle R et al. (2009) IFAP the p.N508S mutation (Aten et al., Building, University College London Genetics syndrome is caused by deficiency in MBTPS2, 2010). It is unclear why this is but it Institute, University College London, London, an intramembrane zinc metalloprotease essen- may be because of differences in UK tial for cholesterol homeostasis and ER stress E-mail: [email protected] response. -

ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 12-2008 ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Yu Fukuda University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Chemicals and Drugs Commons, and the Medical Sciences Commons Recommended Citation Fukuda, Yu , "ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters" (2008). Theses and Dissertations (ETD). Paper 345. http://dx.doi.org/10.21007/etd.cghs.2008.0100. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. ABCB6 Is a Porphyrin Transporter with a Novel Trafficking Signal That Is Conserved in Other ABC Transporters Document Type Dissertation Degree Name Doctor of Philosophy (PhD) Program Interdisciplinary Program Research Advisor John D. Schuetz, Ph.D. Committee Linda Hendershot, Ph.D. James I. Morgan, Ph.D. Anjaparavanda P. Naren, Ph.D. Jie Zheng, Ph.D. DOI 10.21007/etd.cghs.2008.0100 This dissertation is available at UTHSC Digital Commons: https://dc.uthsc.edu/dissertations/345 ABCB6 IS A PORPHYRIN TRANSPORTER WITH A NOVEL TRAFFICKING SIGNAL THAT -

ABCA12 (N-16): Sc-48435
SAN TA C RUZ BI OTEC HNOL OG Y, INC . ABCA12 (N-16): sc-48435 BACKGROUND CHROMOSOMAL LOCATION The ATP binding cassette (ABC) transporters, or traffic ATPases, constitute Genetic locus: ABCA12 (human) mapping to 2q35; Abca12 (mouse) mapping an expansive family of proteins accountable for the transport of a wide to 1 C3. variety of substrates across cell membranes in both prokaryotic and eukary - otic cells. They also aid in the regulation of lipid transport and membrane SOURCE trafficking. ABCA12 (ATP-binding cassette, subfamily A, member 12) contains ABCA12 (N-16) is an affinity purified goat polyclonal antibody raised against two transmembrane (TM) domains, each with six membrane-spanning seg - a peptide mapping within an internal region of ABCA12 of human origin. ments and two nucleotide-binding domains (NBDs), which are located in the cytoplasm. ABCA12 is expressed in normal human keratinocytes (RT-PCR PRODUCT reveals expression in placenta, testis, fetal brain and skin) and is upregulated during keratinization. Immunoelectron microscopy reveals that the ABCA12 Each vial contains 200 µg IgG in 1.0 ml of PBS with < 0.1% sodium azide protein is located in lamellar granules in the upper epidermal keratinocytes and 0.1% gelatin. of human skin. The ABCA12 gene, which synthesizes a 2,595 amino acid Blocking peptide available for competition studies, sc-48435 P, (100 µg protein, may produce an alternative splice variant with an in-frame deletion peptide in 0.5 ml PBS containing < 0.1% sodium azide and 0.2% BSA). leading to truncation of 79 amino acids. APPLICATIONS REFERENCES ABCA12 (N-16) is recommended for detection of ABCA12 of mouse, rat and 1. -

Transcriptional and Post-Transcriptional Regulation of ATP-Binding Cassette Transporter Expression
Transcriptional and Post-transcriptional Regulation of ATP-binding Cassette Transporter Expression by Aparna Chhibber DISSERTATION Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Pharmaceutical Sciences and Pbarmacogenomies in the Copyright 2014 by Aparna Chhibber ii Acknowledgements First and foremost, I would like to thank my advisor, Dr. Deanna Kroetz. More than just a research advisor, Deanna has clearly made it a priority to guide her students to become better scientists, and I am grateful for the countless hours she has spent editing papers, developing presentations, discussing research, and so much more. I would not have made it this far without her support and guidance. My thesis committee has provided valuable advice through the years. Dr. Nadav Ahituv in particular has been a source of support from my first year in the graduate program as my academic advisor, qualifying exam committee chair, and finally thesis committee member. Dr. Kathy Giacomini graciously stepped in as a member of my thesis committee in my 3rd year, and Dr. Steven Brenner provided valuable input as thesis committee member in my 2nd year. My labmates over the past five years have been incredible colleagues and friends. Dr. Svetlana Markova first welcomed me into the lab and taught me numerous laboratory techniques, and has always been willing to act as a sounding board. Michael Martin has been my partner-in-crime in the lab from the beginning, and has made my days in lab fly by. Dr. Yingmei Lui has made the lab run smoothly, and has always been willing to jump in to help me at a moment’s notice. -

The Role of Mitochondrial ATP-Binding Cassette Transporter
The Role of Mitochondrial ATP-Binding Cassette Transporter ABCB6 in Metabolism and Energy Balance By © 2019 Robert T Tessman Submitted to the graduate degree program in Toxicology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Committee Chair: Partha Kasturi, PhD Bruno Hagenbuch, PhD Hao Zhu, PhD Tiangang Li, PhD Luciano DiTacchio, PhD Date Defended: April 19th, 2019Title Page ii The dissertation committee for Robert T Tessman certifies that this is the approved version of the following dissertation: The role of Mitochondrial ATP-Binding Cassette Transporter ABCB6 in Metabolism and Energy Balance Committee Chair: Partha Kasturi, PhD Acceptance Page Date Approved: April 19th, 2019 iii Abstract Obesity and the associated health risks represent a world-wide health and financial crisis. Lack of physical activity combined with excessive caloric intake are the root cause of the problem. Despite the increased advocation for healthy lifestyle choices, the trend has yet to reverse and indeed, seems to be on the rise especially among pre- teens and adolescents, a constituent that had not been previously part of the obesity epidemic. Mitochondria are the “fuel-burners” of the body and like other combustion devices, become inefficient in the context of fuel surplus. Moreover, with chronic over-feeding, the physiological mechanisms that regulate energy balance become permanently dysfunctional leading to the progression of pathologies such as Type II diabetes and cardiovascular disease. Medical and scientific evidence confirms that mitochondria are integral to the responses necessary to adapt to over-nutrition. However, success in mitochondria- based therapies has been extremely limited in the context of metabolic diseases. -

ABCA12 Is the Major Harlequin Ichthyosis Gene Anna C
ORIGINAL ARTICLE ABCA12 Is the Major Harlequin Ichthyosis Gene Anna C. Thomas1, Tom Cullup2, Elizabeth E. Norgett1, Tara Hill3, Stephanie Barton4, Beverly A. Dale5, Eli Sprecher6, Eamonn Sheridan7, Aileen E. Taylor8, Robert S. Wilroy9, Celia DeLozier10, Nigel Burrows11, Helen Goodyear12, Philip Fleckman5, Karen G. Stephens5, Lakshmi Mehta13, Rosemarie M. Watson14, Robert Graham15, Roni Wolf16, Anne Slavotinek17, Madelena Martin17, David Bourn4, Charles A. Mein2, Edel A. O’Toole1 and David P. Kelsell1 Harlequin ichthyosis (HI) is the most severe form of autosomal-recessive, congenital ichthyosis. Affected infants have markedly impaired barrier function and are more susceptible to infection. Abnormalities in the localization of epidermal lipids as well as abnormal lamellar granule formation are features of HI skin. Previously, we and others have shown that mutations in the ABCA12 gene encoding an adenosine triphosphate-binding cassette (ABC) transporter underlie the skin disease HI. In this study, we have sequenced the ABCA12 gene in an additional 14 patients and show that all contain mutations, with the majority being either nonsense substitution or frameshift mutations. Eleven HI patients had bi-allelic ABCA12 mutations, whereas in the remaining three HI patients in this study, ABCA12 mutations were detected on only one allele by sequencing. In addition, the one patient from the previous study where no sequence mutations were detected was screened for heterozygous deletions. A combination of oligonucleotide arrays, multiplex PCR analysis -

Interindividual Differences in the Expression of ATP-Binding
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2018/02/02/dmd.117.079061.DC1 1521-009X/46/5/628–635$35.00 https://doi.org/10.1124/dmd.117.079061 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:628–635, May 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Special Section on Transporters in Drug Disposition and Pharmacokinetic Prediction Interindividual Differences in the Expression of ATP-Binding Cassette and Solute Carrier Family Transporters in Human Skin: DNA Methylation Regulates Transcriptional Activity of the Human ABCC3 Gene s Tomoki Takechi, Takeshi Hirota, Tatsuya Sakai, Natsumi Maeda, Daisuke Kobayashi, and Ichiro Ieiri Downloaded from Department of Clinical Pharmacokinetics, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan (T.T., T.H., T.S., N.M., I.I.); Drug Development Research Laboratories, Kyoto R&D Center, Maruho Co., Ltd., Kyoto, Japan (T.T.); and Department of Clinical Pharmacy and Pharmaceutical Care, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan (D.K.) Received October 19, 2017; accepted January 30, 2018 dmd.aspetjournals.org ABSTRACT The identification of drug transporters expressed in human skin and levels. ABCC3 expression levels negatively correlated with the methylation interindividual differences in gene expression is important for understanding status of the CpG island (CGI) located approximately 10 kilobase pairs the role of drug transporters in human skin. In the present study, we upstream of ABCC3 (Rs: 20.323, P < 0.05). The reporter gene assay revealed evaluated the expression of ATP-binding cassette (ABC) and solute carrier a significant increase in transcriptional activity in the presence of CGI. -

Whole-Exome Sequencing Identifies Novel Mutations in ABC Transporter
Liu et al. BMC Pregnancy and Childbirth (2021) 21:110 https://doi.org/10.1186/s12884-021-03595-x RESEARCH ARTICLE Open Access Whole-exome sequencing identifies novel mutations in ABC transporter genes associated with intrahepatic cholestasis of pregnancy disease: a case-control study Xianxian Liu1,2†, Hua Lai1,3†, Siming Xin1,3, Zengming Li1, Xiaoming Zeng1,3, Liju Nie1,3, Zhengyi Liang1,3, Meiling Wu1,3, Jiusheng Zheng1,3* and Yang Zou1,2* Abstract Background: Intrahepatic cholestasis of pregnancy (ICP) can cause premature delivery and stillbirth. Previous studies have reported that mutations in ABC transporter genes strongly influence the transport of bile salts. However, to date, their effects are still largely elusive. Methods: A whole-exome sequencing (WES) approach was used to detect novel variants. Rare novel exonic variants (minor allele frequencies: MAF < 1%) were analyzed. Three web-available tools, namely, SIFT, Mutation Taster and FATHMM, were used to predict protein damage. Protein structure modeling and comparisons between reference and modified protein structures were performed by SWISS-MODEL and Chimera 1.14rc, respectively. Results: We detected a total of 2953 mutations in 44 ABC family transporter genes. When the MAF of loci was controlled in all databases at less than 0.01, 320 mutations were reserved for further analysis. Among these mutations, 42 were novel. We classified these loci into four groups (the damaging, probably damaging, possibly damaging, and neutral groups) according to the prediction results, of which 7 novel possible pathogenic mutations were identified that were located in known functional genes, including ABCB4 (Trp708Ter, Gly527Glu and Lys386Glu), ABCB11 (Gln1194Ter, Gln605Pro and Leu589Met) and ABCC2 (Ser1342Tyr), in the damaging group. -

ABCA7 and Pathogenic Pathways of Alzheimer's Disease
brain sciences Review ABCA7 and Pathogenic Pathways of Alzheimer’s Disease Tomonori Aikawa, Marie-Louise Holm ID and Takahisa Kanekiyo * Department of Neuroscience, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA; [email protected] (T.A.); [email protected] (M.-L.H.) * Correspondence: [email protected]; Tel.: +1-904-953-2483 Received: 28 December 2017; Accepted: 3 February 2018; Published: 5 February 2018 Abstract: The ATP-binding cassette (ABC) reporter family functions to regulate the homeostasis of phospholipids and cholesterol in the central nervous system, as well as peripheral tissues. ABCA7 belongs to the A subfamily of ABC transporters, which shares 54% sequence identity with ABCA1. While ABCA7 is expressed in a variety of tissues/organs, including the brain, recent genome-wide association studies (GWAS) have identified ABCA7 gene variants as susceptibility loci for late-onset Alzheimer’s disease (AD). More important, subsequent genome sequencing analyses have revealed that premature termination codon mutations in ABCA7 are associated with the increased risk for AD. Alzheimer’s disease is a progressive neurodegenerative disease and the most common cause of dementia, where the accumulation and deposition of amyloid-β (Aβ) peptides cleaved from amyloid precursor protein (APP) in the brain trigger the pathogenic cascade of the disease. In consistence with human genetic studies, increasing evidence has demonstrated that ABCA7 deficiency exacerbates Aβ pathology using in vitro and in vivo models. While ABCA7 has been shown to mediate phagocytic activity in macrophages, ABCA7 is also involved in the microglial Aβ clearance pathway. Furthermore, ABCA7 deficiency results in accelerated Aβ production, likely by facilitating endocytosis and/or processing of APP. -

The ABCA4 2588G>C Stargardt Mutation
European Journal of Human Genetics (2002) 10, 197 ± 203 ã 2002 Nature Publishing Group All rights reserved 1018-4813/02 $25.00 www.nature.com/ejhg ARTICLE The ABCA4 2588G4C Stargardt mutation: single origin and increasing frequency from South-West to North-East Europe Alessandra Maugeri*,1, Kris Flothmann2, Nadine Hemmrich3, Sofie Ingvast4, Paula Jorge5, Eva Paloma6, Reshma Patel7, Jean-Michel Rozet8, Jaana Tammur9,10, Francesco Testa11, Susana Balcells6, Alan C Bird12, Han G Brunner1, Carel B Hoyng13, Andres Metspalu9, Francesca Simonelli11, Rando Allikmets10,14, Shomi S Bhattacharya7, Michele D'Urso15, Roser GonzaÁlez-Duarte6, Josseline Kaplan8, Gerard J te Meerman16, RosaÂrio Santos5, Marianne Schwartz17, Guy Van Camp2, Claes Wadelius4, Bernhard HF Weber3 and Frans PM Cremers1 1Department of Human Genetics, University Medical Center Nijmegen, Nijmegen, The Netherlands; 2Department of Medical Genetics, University of Antwerp, Universiteitsplein 1, 2610 Antwerp, Belgium; 3Institute of Human Genetics, Biocenter, Julius-Maximilians-University of Wuerzburg, Wuerzburg, Germany; 4Department of Genetics and Pathology, Rudbeck Laboratory, Uppsala University, Uppsala, Sweden; 5Unidade de GeneÁtica Molecular, Instituto de GeneÁtica MeÁdica, PracËa Pedro Nunes 88, 4050-466 Porto, Portugal; 6Departament de GeneÁtica, Universitat de Barcelona, Avda. Diagonal 645, 08028-Barcelona, Spain; 7Department of Molecular Genetics, Institute of Ophthalmology, University College London, Bath Street, London EC1V 9EL, UK; 8Laboratoire de Recherches sur les Handicaps, -
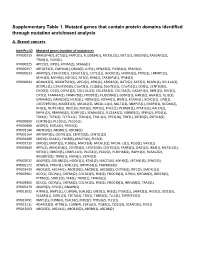
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

The Role of ABCA7 in Alzheimer's Disease: Evidence from Genomics
Acta Neuropathologica https://doi.org/10.1007/s00401-019-01994-1 REVIEW The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics Arne De Roeck1,2 · Christine Van Broeckhoven1,2 · Kristel Sleegers1,2 Received: 1 February 2019 / Revised: 18 March 2019 / Accepted: 18 March 2019 © The Author(s) 2019 Abstract Genome-wide association studies (GWAS) originally identifed ATP-binding cassette, sub-family A, member 7 (ABCA7), as a novel risk gene of Alzheimer’s disease (AD). Since then, accumulating evidence from in vitro, in vivo, and human-based studies has corroborated and extended this association, promoting ABCA7 as one of the most important risk genes of both early-onset and late-onset AD, harboring both common and rare risk variants with relatively large efect on AD risk. Within this review, we provide a comprehensive assessment of the literature on ABCA7, with a focus on AD-related human -omics studies (e.g. genomics, transcriptomics, and methylomics). In European and African American populations, indirect ABCA7 GWAS associations are explained by expansion of an ABCA7 variable number tandem repeat (VNTR), and a common pre- mature termination codon (PTC) variant, respectively. Rare ABCA7 PTC variants are strongly enriched in AD patients, and some of these have displayed inheritance patterns resembling autosomal dominant AD. In addition, rare missense variants are more frequent in AD patients than healthy controls, whereas a common ABCA7 missense variant may protect from disease. Methylation at several CpG sites in the ABCA7 locus is signifcantly associated with AD. Furthermore, ABCA7 contains many diferent isoforms and ABCA7 splicing has been shown to associate with AD.