Understanding the Breeding Systems of Cakile Edentula, Cakile Maritima
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California
RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California Prepared for: Monterey Peninsula Regional Park District 4860 Carmel Valley Road Carmel, CA 93923 Prepared by: Burleson Consulting Inc. 1900 Garden Road, Suite 210 Monterey, CA 93940 March 2021 This page intentionally left blank Restoration Action Plan, Marina Dunes Preserve CONTENTS CONTENTS ..........................................................................................................................................i APPENDICES ...................................................................................................................................... ii ACRONYMS AND ABBREVIATIONS ..................................................................................................... iii 1. INTRODUCTION ...................................................................................................................... 1 1.1 Setting ........................................................................................................................................... 1 1.2 Purpose ......................................................................................................................................... 1 1.3 Approach ....................................................................................................................................... 2 2. UPDATED BEST MANAGEMENT PRACTICES .............................................................................. 3 2.1 Weed Eradication and Control ..................................................................................................... -

Macroevolutionary Patterns of Glucosinolate Defense and Tests of Defense-Escalation and Resource Availability Hypotheses
Research Macroevolutionary patterns of glucosinolate defense and tests of defense-escalation and resource availability hypotheses N. Ivalu Cacho1,2, Daniel J. Kliebenstein3,4 and Sharon Y. Strauss1 1Center for Population Biology, and Department of Evolution of Ecology, University of California, One Shields Avenue, Davis, CA 95616, USA; 2Instituto de Biologıa, Universidad Nacional Autonoma de Mexico, Circuito Exterior, Ciudad Universitaria, 04510 Mexico City, Mexico; 3Department of Plant Sciences, University of California. One Shields Avenue, Davis, CA 95616, USA; 4DynaMo Center of Excellence, University of Copenhagen, Thorvaldsensvej 40, DK-1871 Frederiksberg C, Denmark Summary Author for correspondence: We explored macroevolutionary patterns of plant chemical defense in Streptanthus (Brassi- N. Ivalu Cacho caceae), tested for evolutionary escalation of defense, as predicted by Ehrlich and Raven’s Tel: +1 530 304 5391 plant–herbivore coevolutionary arms-race hypothesis, and tested whether species inhabiting Email: [email protected] low-resource or harsh environments invest more in defense, as predicted by the resource Received: 13 April 2015 availability hypothesis (RAH). Accepted: 8 June 2015 We conducted phylogenetically explicit analyses using glucosinolate profiles, soil nutrient analyses, and microhabitat bareness estimates across 30 species of Streptanthus inhabiting New Phytologist (2015) 208: 915–927 varied environments and soils. doi: 10.1111/nph.13561 We found weak to moderate phylogenetic signal in glucosinolate classes -
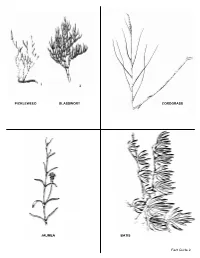
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Brassicaceae) in Australia
Cunninghamia Date of Publication: 26/08/2013 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) Reassessment of the invasion history of two species of Cakile (Brassicaceae) in Australia Roger D. Cousens, Peter K. Ades, Mohsen B. Mesgaran and Sara Ohadi Melbourne School of Land & Environment, The University of Melbourne, Victoria, 3010, AUSTRALIA Abstract: In this paper we revisit the invasion history of two species of Cakile in Australia. Cakile edentula subsp. edentula arrived in the mid 19th Century and spread into coastal strandline habitat from the southeast towards the west and to the north; Cakile maritima arrived in the late 19th Century and has replaced Cakile edentula over much of the range. While Cakile edentula is morphologically quite uniform, the great variation within Cakile maritima has confused field ecologists. Using herbarium records we update previous accounts of the spread of the species and report on field surveys that determined their current geographic overlap in Tasmania and in northern New South Wales/southern Queensland. We examine regional morphological variation within Cakile maritima using the national herbaria collections and variation within new population samples. We support previous interpretations that Cakile maritima has been introduced on more than one occasion from morphologically distinct races, resulting in regional variation within Australia and high variability within populations in the south-east. Western Australian populations appear distinct and probably did not initiate those in the east; we consider that eastern populations are likely to be a mix of Cakile maritima subsp. maritima from the Mediterranean and Cakile maritima subsp. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Taxa Named in Honor of Ihsan A. Al-Shehbaz
TAXA NAMED IN HONOR OF IHSAN A. AL-SHEHBAZ 1. Tribe Shehbazieae D. A. German, Turczaninowia 17(4): 22. 2014. 2. Shehbazia D. A. German, Turczaninowia 17(4): 20. 2014. 3. Shehbazia tibetica (Maxim.) D. A. German, Turczaninowia 17(4): 20. 2014. 4. Astragalus shehbazii Zarre & Podlech, Feddes Repert. 116: 70. 2005. 5. Bornmuellerantha alshehbaziana Dönmez & Mutlu, Novon 20: 265. 2010. 6. Centaurea shahbazii Ranjbar & Negaresh, Edinb. J. Bot. 71: 1. 2014. 7. Draba alshehbazii Klimeš & D. A. German, Bot. J. Linn. Soc. 158: 750. 2008. 8. Ferula shehbaziana S. A. Ahmad, Harvard Pap. Bot. 18: 99. 2013. 9. Matthiola shehbazii Ranjbar & Karami, Nordic J. Bot. doi: 10.1111/j.1756-1051.2013.00326.x, 10. Plocama alshehbazii F. O. Khass., D. Khamr., U. Khuzh. & Achilova, Stapfia 101: 25. 2014. 11. Alshehbazia Salariato & Zuloaga, Kew Bulletin …….. 2015 12. Alshehbzia hauthalii (Gilg & Muschl.) Salariato & Zuloaga 13. Ihsanalshehbazia Tahir Ali & Thines, Taxon 65: 93. 2016. 14. Ihsanalshehbazia granatensis (Boiss. & Reuter) Tahir Ali & Thines, Taxon 65. 93. 2016. 15. Aubrieta alshehbazii Dönmez, Uǧurlu & M.A.Koch, Phytotaxa 299. 104. 2017. 16. Silene shehbazii S.A.Ahmad, Novon 25: 131. 2017. PUBLICATIONS OF IHSAN A. AL-SHEHBAZ 1973 1. Al-Shehbaz, I. A. 1973. The biosystematics of the genus Thelypodium (Cruciferae). Contrib. Gray Herb. 204: 3-148. 1977 2. Al-Shehbaz, I. A. 1977. Protogyny, Cruciferae. Syst. Bot. 2: 327-333. 3. A. R. Al-Mayah & I. A. Al-Shehbaz. 1977. Chromosome numbers for some Leguminosae from Iraq. Bot. Notiser 130: 437-440. 1978 4. Al-Shehbaz, I. A. 1978. Chromosome number reports, certain Cruciferae from Iraq. -

Larkspur Poisoning: Toxicology and Alkaloid Structure–Activity Relationshipsଝ K.E
Biochemical Systematics and Ecology 30 (2002) 113–128 www.elsevier.com/locate/biochemsyseco Larkspur poisoning: toxicology and alkaloid structure–activity relationshipsଝ K.E. Panter a,*, G.D. Manners b, B.L. Stegelmeier a, S. Lee a, D.R. Gardner a, M.H. Ralphs a, J.A. Pfister a, L.F. James a a USDA-ARS Poisonous Plant Research Laboratory, 1150 E. 1400 N., Logan, UT 84341, USA b USDA-ARS Western Regional Research Center, 800 Buchanan Street, Albany, CA 94710, USA Received 4 January 2001; accepted 1 May 2001 Abstract Systematic approaches to taxonomic classifications of the tall larkspur spp. have been developed using traditional chemical methods to profile alkaloids, comparison of relative tox- icity of individual alkaloids, plant morphology/taxonomy and molecular genetics. Using these methods (papers published in this series) toxicology of three distinct species of tall larkspurs including Delphinium glaucum, Delphinium barbeyi and Delphinium occidentale is described. Tall larkspurs (Delphinium spp.) continue to be the most serious cause of cattle losses on mountain rangelands in the western US. Over 40 norditerpenoid alkaloids have been reported in species of larkspurs and toxicology data on 25 of these have been reported by the authors. These alkaloids can be classified into three general types based on their structural character- istics and toxicity: the N-(methylsuccinyl) anthranoyllycoctonine (MSAL)-type, having high toxicity; the lycoctonine-type, with moderate toxicity; and the 7,8-methylenedioxylycoctonine (MDL)-type, of low toxicity. The structural importance of the methylsuccinimido anthranilic acid ester group at the C18 position is evident in the high toxicity of MSAL alkaloids, parti- cularly methyllycaconitine (MLA), Nudicauline (NUD) and 14-deacetylnudicauline (14-DAN). -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

2004 Vegetation Classification and Mapping of Peoria Wildlife Area
Vegetation classification and mapping of Peoria Wildlife Area, South of New Melones Lake, Tuolumne County, California By Julie M. Evens, Sau San, and Jeanne Taylor Of California Native Plant Society 2707 K Street, Suite 1 Sacramento, CA 95816 In Collaboration with John Menke Of Aerial Information Systems 112 First Street Redlands, CA 92373 November 2004 Table of Contents Introduction.................................................................................................................................................... 1 Vegetation Classification Methods................................................................................................................ 1 Study Area ................................................................................................................................................. 1 Figure 1. Survey area including Peoria Wildlife Area and Table Mountain .................................................. 2 Sampling ................................................................................................................................................ 3 Figure 2. Locations of the field surveys. ....................................................................................................... 4 Existing Literature Review ......................................................................................................................... 5 Cluster Analyses for Vegetation Classification ......................................................................................... -

The Brassicaceae of Ohio
THE BRASSICACEJE OF OHIO. EMMA E. LAUGHLIN. Brassicaceae. Mustard Family. Herbs, with watery sap of a pungent taste, not poisonous; with alternate, exstipulate leaves, usually large at the base of the stem and intergrading in form to the top of the stem. Flowers hypogynous, bisporangiate, usually isobilateral, appear- ing actinomorphic, regular, usually with glands, in racemes, short at first and elongating, or in corymbs; calyx of 4 sepals, decidu- ous, rarely persistent; corolla choripetalous, tetramerous, cruci- form; stamens 6, tetradynamous, rarely 4 or 2; ovulary com- pound, bilocular, the parietal placentae connected by a thin septum from which the valves separate when ripe; ovules 2 to several, campylotropous; fruit a silique if longer than broad, or a silicle if short, generally with 2 cavities, sometimes uni- locular, dehiscent or in a few genera indehiscent; endosperm scanty; cotyledons accumbent, incumbent or conduplicate. SYNOPSIS. I. Pod usually not more than twice as long as wide (a silicle); cotyledons accum- bent or incumbent. A. Pods more or less flattened parallel to the broad partition, dehiscent; cotyledons accumbent; leaves not lobed. 1. Pubescence stellate or of forked hairs. Berteroa, Koniga, Alyssum, Draba. 2. Pubescence of simple hairs or wanting; pods very broad and flat; leaves opposite. LUNARIE^E. Lunaria. B. Pods flattened at right angles to the partition or not flattened. 1. Pubescence of forked hairs; cotyledons incumbent. CAMELINE^E. Camelina, Bursa, Neslia. 2. Pubescence of simple hairs or wanting. a. Pod scarcely or not at all flattened; cotyledous accumbent. COCHLEARIE^E. Armoracia, Neobeckia, Sisymbrium, Radicula. b. Pods strongly flattened at right angles to the narrow partition.