The Supernumerary Segment Systems of Rumex Acetosa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Cory Family of Tresparret and Jersey
The Cory Family of Tresparret and Jersey R.J, Champ 2009 Contents Introduction .................................................................................................................................................3 Robert & Alice .............................................................................................................................................6 Alice ............................................................................................................................................................11 William, Son of Robert. ...........................................................................................................................17 Jane ..............................................................................................................................................................20 Mary ............................................................................................................................................................24 Richard ........................................................................................................................................................25 William Son of Richard ............................................................................................................................30 John, Son of Richard .................................................................................................................................31 Moses, Son of Richard ..............................................................................................................................33 -
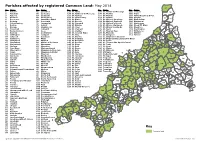
Parish Boundaries
Parishes affected by registered Common Land: May 2014 94 No. Name No. Name No. Name No. Name No. Name 1 Advent 65 Lansall os 129 St. Allen 169 St. Martin-in-Meneage 201 Trewen 54 2 A ltarnun 66 Lanteglos 130 St. Anthony-in-Meneage 170 St. Mellion 202 Truro 3 Antony 67 Launce lls 131 St. Austell 171 St. Merryn 203 Tywardreath and Par 4 Blisland 68 Launceston 132 St. Austell Bay 172 St. Mewan 204 Veryan 11 67 5 Boconnoc 69 Lawhitton Rural 133 St. Blaise 173 St. M ichael Caerhays 205 Wadebridge 6 Bodmi n 70 Lesnewth 134 St. Breock 174 St. Michael Penkevil 206 Warbstow 7 Botusfleming 71 Lewannick 135 St. Breward 175 St. Michael's Mount 207 Warleggan 84 8 Boyton 72 Lezant 136 St. Buryan 176 St. Minver Highlands 208 Week St. Mary 9 Breage 73 Linkinhorne 137 St. C leer 177 St. Minver Lowlands 209 Wendron 115 10 Broadoak 74 Liskeard 138 St. Clement 178 St. Neot 210 Werrington 211 208 100 11 Bude-Stratton 75 Looe 139 St. Clether 179 St. Newlyn East 211 Whitstone 151 12 Budock 76 Lostwithiel 140 St. Columb Major 180 St. Pinnock 212 Withiel 51 13 Callington 77 Ludgvan 141 St. Day 181 St. Sampson 213 Zennor 14 Ca lstock 78 Luxul yan 142 St. Dennis 182 St. Stephen-in-Brannel 160 101 8 206 99 15 Camborne 79 Mabe 143 St. Dominic 183 St. Stephens By Launceston Rural 70 196 16 Camel ford 80 Madron 144 St. Endellion 184 St. Teath 199 210 197 198 17 Card inham 81 Maker-wi th-Rame 145 St. -

February 2020 No.473 50P to Non Residents
February 2020 No.473 50p to Non Residents The Compass from The Swedish Brigantine ‘Wilhelm’ Wrecked on 29th January 1894 see pages 14 & 16 for the full story 1 Events 2 CONTENTS PAGE Editorial Age Concern 12 Tony was asking what I would like Bottreaux Surgery 10 for our up and coming anniversary. Business Index 38 I said I wanted something shiny Competition 16 that goes from 0 to 150 in about 3 Compass Story 14,16 seconds. Crackington Shop 9 So he bought me some bathroom Churches 22,32 scales! Directory of Clubs 37 Happy Valentine’s Day Events and Reviews 2,5,39,40 And be ready for Spring! Hedges 26 “May the weather be with you” Interests 18 (quote from Radio 4 Gardener’s Notices 7,24,30 Question Time) Parish Council 34,35 Heather Parish Diary 20,21 Green Quiz 28 St Gennys Cricketers 10 What to do in Garden 22 The Deadline for submissions to the next Gazette is end of day 17th of this month email:[email protected] Your Editors are: Heather Smith 01840 230976 Margaret Kirkwood 01840 230911 The St Gennys Gazette exists for the benefit of St Gennys Parish and its residents. It aims to provide an information service to publicise events and activities in St Gennys and its neighbouring parishes and publish Parish topics of interest. Publishing includes printed, digital and online for- mats. It takes no editorial positions. The Editors may edit articles, letters, adverts or any other content submitted to the Gazette. They reserve the right not to publish anything that they judge to be counter to the intentions of the Gazette or generally inappropriate for publication. -

St Gennys School Admissions
St Gennys School Admissions Transcribed from LDS Film No. 1471875 by Phil Rodda N.B. Exempt refers to "Exempt from Religious Instruction" Admission Forename(s) Surname DoB Parent/ Occupation Residence Exempt Last School Standard Date of Register Notes Transcriber Notes No. Date Year Guardian leaving 105‐Feb 1877 Enoch HEAL 01‐04‐1863 James Farmer St Gennys No St Gennys 13/12/1878 205‐Feb 1877 Ernest MARSHALL 14‐09‐1865 Frank Carpenter Catch Gate, St Gennys No St Juliott II 1879 305‐Feb 1877 William MOYSE 31‐08‐1867 Henry Farmer Pencuke No St Gennys 405‐Feb 1877 Clara FOLLY 27‐01‐1870 Thomas Shoemaker Higher Crackington No St Gennys 505‐Feb 1877 Thirza FOLLY 09‐03‐1866 Thomas Shoemaker Higher Crackington No St Gennys 605‐Feb 1877 William H. STONE 29‐05‐1868 John Labourer Sweets No St Gennys 705‐Feb 1877 Arthur STONE 29‐05‐1869 John Labourer Sweets No St Gennys 805‐Feb 1877 William G. JEWEL 22‐09‐1862 George Labourer Cleave No St Juliott II 08/05/1878 Left 905‐Feb 1877 William EDWARDS 26‐12‐1866 William Labourer Trespaddock No St Gennys 20/05/1880 10 05‐Feb 1877 William H. GREENWOOD 13‐03‐1869 Thomas Labourer Wood Park No St Gennys 11 05‐Feb 1877 Mary E. GREENWOOD 14‐02‐1866 Thomas Labourer Wood Park No St Gennys 19/12/1879 12 05‐Feb 1877 Thomas HICKS Dec‐1869 John Farmer Dizard No St Gennys 13 05‐Feb 1877 Thomas BONEY 21‐08‐1868 Thomas Labourer Small Hill Burrow No St Gennys 14 05‐Feb 1877 Mary E. -
![Directory. St. Gennys. [Cornwall.]](https://docslib.b-cdn.net/cover/2939/directory-st-gennys-cornwall-342939.webp)
Directory. St. Gennys. [Cornwall.]
DIRECTORY. 113 ST. GENNYS. [CORNWALL.] ST. GENNYS is a parish, 16~ miles north-west from Lord RoUe are lords of the manor, and, with Lewis William Launceston, 22 north from Bodmin, Ill south-south-west Buck, M,P., and Robert Chichester, Esq., are chief land from Stratton, and 10 north from Camelford, in the east owners. The chief crops are corn. division of Cornwall, Lesnewth Hundred, Stratton Union, The following are hamlets, with their distances from the Trigg Major deanery, Cornwall arch deaconry, and Exeter chnreh :-RoscARE,ll miles; Trencrick, 2~ miles; Coxford, bishopric; it is situated on the north coast of Cornwall. l~alf a mile ; Cracking ton Haven, three-quarters of a mile; The church of St. Genesius is an old stone building, in the Dysart, 2 miles; Sweets, ll miles; Hill, 2 miles; Pen early English style of architecture; has a nave, aisles, kuke, 2 miles, chancel, porch, low tower, 4 bells, and font. The living is The following are adjacent places, with their distances a vicarage, worth £160 yearly, with residence and 27 from the church :-TRENCRICK, i>! miles; Hole, Il miles; acres of glebe land, in the gift of the Earl of St. Germans; West Dysart, 2 miles; Church Town, adjoining Baypark, the Rev. John A thanasius Herring Laffer, B.A., is the in 2 miles; Halligather, 1 ~ miles; Treleigh, 2 miles; Middle cumbent. There is a chapel for Association Methodists. Crackington, I mile; Hentervean, 2 miles; Whitemoor, There is a parochial school in connexion with the church. I!- miles; Flanders, 1 mile; Penruke, 2 miles; Ludon, A fair is held on July 16th yearly, for cattle and sheep. -

CORNWALL Hender W. St. Thomas Hill, Launceston Hicks S
190 CORNWALL POST FARMERs-continued. Hender W. St. Thomas hill, Launceston Hicks S. Lewanick, Launceston Hawken G.L. Dannonchapple,f:t.Teath, Hendy A. Trebell, Lanivet, Bodmin Hicks T. Carn, Lelant, Hay le Camelford Hendy E. Trebell, Lanivet, Bodmin Hicks T. Chynalls, St. Paul, Penzance Hawken H. Trefresa, Wadebridge Hendy H. Carmina, Mawgan, Helston Hicks T. Sancreed, Peuzance *Haw ken J.Penrose,St.Ervan, Padstow Hendy J. Trethurffe, Ladock,Grmpound Hicks T. Prideaux, Luxulion, Bodmin Hawken J. Treginnegar, Padstow Hendy J. Frogwell, Callington Hicks T. St. Autbony, Tre~ony HawkenJ.Treburrick,St.Ervan,Padstow Hendy J. Skewes, Cury, Helston Hicks T. Lanivet, Bodmin Haw ken J. jun. Penro~e, Pads tow Hendy J. Frowder, Mullion, Helston Hick;~ T. St. Gerrans, Gram pound Hawken N. Treore, Wadebridge Hendy M. Swyna, Gunwallot>, Helston Hicks T. St. Gennys, Camt>lford Haw ken P. Longcarne, Camelt'ord Hendy S. GunwalloP, Helston Hicks T.jun. Tregarneer,St.Colmb.Major Haw ken P.Tre~wyn, St. Ervan,Padstow Hendy T. Lizard, Helston Hicks W. Clift' farm, Anthony Haw ken R. Stanon,St.Breward, Bodmin Hendy W. Chimber, Gunwalloe,Helston Hicks W. St. Agnes, Scilly HawkenR.G.Trt-gwormond,Wadebrilige Hendy W. Mullion, Ht-lston Hicks W. Newlyn East, Grampound HawkenS.Low.Nankelly,St.ColumhMjr Ht>ndy W. PolJ(reen, Cury, Helston Hicks W. PencrebPr farm, Caliington Hawken T. Hale, St. Kew, Wadebridge Hendy W. Polgreen,Gunwalloe, Helston Hicks W. Fowey, Lostwithiel Haw ken T. Heneward, Bolimin Hermah H. Penare, Gorran, St. A ustell Hicks W. St. Agnes, Scilly Haw ken T. Trevorrick, St.lssry ,Bodmin Hennah T. -

The Legendary Lore of the Holy Wells of England
'? '/-'#'•'/ ' ^7 f CX*->C5CS- '^ OF CP^ 59§70^ l-SSi"-.". -,, 3 ,.. -SJi f, THE LEGENDARY LORE OF THE HOL Y WELLS OF ENGLAND. : THE LEGENDARY LORE ' t\Q OF THE ~ 1 T\ I Holy Wells of England: INCLUDING IRfpers, Xaftes, ^fountains, ant) Springs. COPIOUSLY ILLUSTRATED BY CURIOUS ORIGINAL WOODCUTS. ROBERT CHARLES HOPE, F.S.A., F.R.S.L., PETERHOUSE, CAMBRIDGE; LINCOLN'S INN; MEMBER,OF THE COUNCIL OF THE EAST RIDING OF YORKSHIRE ANTIQUARIAN SOCIETY, AUTHOR OF "a GLOSSARY OF DIALECTAL PLACE-NOMENCLATURE," " AN INVENTORY OF THE CHURCH PLATE IN RUTLAND," "ENGLISH GOLDSMITHS," " THE LEPER IN ENGLAND AND ENGLISH LAZAR-HOUSES ;" EDITOR OF BARNABE GOOGE'S " POPISH KINGDOME." LONDON ELLIOT STOCK, 62, PATERNOSTER ROW, E.C. 1893. PREFACE, THIS collection of traditionary lore connected with the Holy Wells, Rivers, Springs, and Lakes of England is the first systematic attempt made. It has been said there is no book in any language which treats of Holy Wells, except in a most fragmentary and discursive manner. It is hoped, therefore, that this may prove the foundation of an exhaustive work, at some future date, by a more competent hand. The subject is almost inexhaustible, and, at the same time, a most interesting one. There is probably no superstition of bygone days that has held the minds of men more tenaciously than that of well-worship in its broadest sense, "a worship simple and more dignified than a senseless crouching before idols." An honest endeavour has been made to render the work as accurate as possible, and to give the source of each account, where such could be ascertained. -

Cornwall Council Altarnun Parish Council
CORNWALL COUNCIL THURSDAY, 4 MAY 2017 The following is a statement as to the persons nominated for election as Councillor for the ALTARNUN PARISH COUNCIL STATEMENT AS TO PERSONS NOMINATED The following persons have been nominated: Decision of the Surname Other Names Home Address Description (if any) Returning Officer Baker-Pannell Lisa Olwen Sun Briar Treween Altarnun Launceston PL15 7RD Bloomfield Chris Ipc Altarnun Launceston Cornwall PL15 7SA Branch Debra Ann 3 Penpont View Fivelanes Launceston Cornwall PL15 7RY Dowler Craig Nicholas Rivendale Altarnun Launceston PL15 7SA Hoskin Tom The Bungalow Trewint Marsh Launceston Cornwall PL15 7TF Jasper Ronald Neil Kernyk Park Car Mechanic Tredaule Altarnun Launceston Cornwall PL15 7RW KATE KENNALLY Dated: Wednesday, 05 April, 2017 RETURNING OFFICER Printed and Published by the RETURNING OFFICER, CORNWALL COUNCIL, COUNCIL OFFICES, 39 PENWINNICK ROAD, ST AUSTELL, PL25 5DR CORNWALL COUNCIL THURSDAY, 4 MAY 2017 The following is a statement as to the persons nominated for election as Councillor for the ALTARNUN PARISH COUNCIL STATEMENT AS TO PERSONS NOMINATED The following persons have been nominated: Decision of the Surname Other Names Home Address Description (if any) Returning Officer Kendall Jason John Harrowbridge Hill Farm Commonmoor Liskeard PL14 6SD May Rosalyn 39 Penpont View Labour Party Five Lanes Altarnun Launceston Cornwall PL15 7RY McCallum Marion St Nonna's View St Nonna's Close Altarnun PL15 7RT Richards Catherine Mary Penpont House Altarnun Launceston Cornwall PL15 7SJ Smith Wes Laskeys Caravan Farmer Trewint Launceston Cornwall PL15 7TG The persons opposite whose names no entry is made in the last column have been and stand validly nominated. -

1859 Cornwall Quarter Sessions & Assizes
1859 Cornwall Quarter Sessions & Assizes Table of Contents 1. Epiphany Sessions ...................................................................................................................... 1 2. Lent Assizes .............................................................................................................................. 24 3. Easter Sessions ........................................................................................................................ 42 4. Midsummer Sessions 1859 ...................................................................................................... 51 5. Summer Assizes ....................................................................................................................... 76 6. Michaelmas Sessions ............................................................................................................. 116 ========== Royal Cornwall Gazette, Friday January 7, 1859 1. Epiphany Sessions These sessions opened at the County Hall, Bodmin, on Tuesday the 4th inst., before the following Magistrates:— Sir Colman Rashleigh, Bart., John Jope Rogers, Esq., Chairmen. C. B. Graves Sawle, Esq., Lord Vivian. Thomas Hext, Esq. Hon. G.M. Fortescue. F.M. Williams, Esq. N. Kendall, Esq., M.P. H. Thomson, Esq. T. J. Agar Robartes, Esq., M.P. J. P. Magor, Esq. R. Davey, Esq., M.P. R. G. Bennet, Esq. J. St. Aubyn, Esq., M.P. Thomas Paynter, Esq. J. King Lethbridge, Esq. R. G. Lakes, Esq. W. H. Pole Carew, Esq. J. T. H. Peter, Esq. J. Tremayne, Esq. C. A. Reynolds, Esq. F. Rodd, -

Cornwall Local Plan: Community Network Area Sections
Planning for Cornwall Cornwall’s future Local Plan Strategic Policies 2010 - 2030 Community Network Area Sections www.cornwall.gov.uk Dalghow Contents 3 Community Networks 6 PP1 West Penwith 12 PP2 Hayle and St Ives 18 PP3 Helston and South Kerrier 22 PP4 Camborne, Pool and Redruth 28 PP5 Falmouth and Penryn 32 PP6 Truro and Roseland 36 PP7 St Agnes and Perranporth 38 PP8 Newquay and St Columb 41 PP9 St Austell & Mevagissey; China Clay; St Blazey, Fowey & Lostwithiel 51 PP10 Wadebridge and Padstow 54 PP11 Bodmin 57 PP12 Camelford 60 PP13 Bude 63 PP14 Launceston 66 PP15 Liskeard and Looe 69 PP16 Caradon 71 PP17 Cornwall Gateway Note: Penzance, Hayle, Helston, Camborne Pool Illogan Redruth, Falmouth Penryn, Newquay, St Austell, Bodmin, Bude, Launceston and Saltash will be subject to the Site Allocations Development Plan Document. This document should be read in conjunction with the Cornwall Local Plan: Strategic Policies 2010 - 2030 Community Network Area Sections 2010-2030 4 Planning for places unreasonably limiting future opportunity. 1.4 For the main towns, town frameworks were developed providing advice on objectives and opportunities for growth. The targets set out in this plan use these as a basis for policy where appropriate, but have been moderated to ensure the delivery of the wider strategy. These frameworks will form evidence supporting Cornwall Allocations Development Plan Document which will, where required, identify major sites and also Neighbourhood Development Plans where these are produced. Town frameworks have been prepared for; Bodmin; Bude; Camborne-Pool-Redruth; Falmouth Local objectives, implementation & Penryn; Hayle; Launceston; Newquay; Penzance & Newlyn; St Austell, St Blazey and Clay Country and monitoring (regeneration plan) and St Ives & Carbis Bay 1.1 The Local Plan (the Plan) sets out our main 1.5 The exception to the proposed policy framework planning approach and policies for Cornwall. -

Election of Parish Councillors for Altarnun Parish Council on Thursday 6 May 2021
RETURN OF RESULT OF UNCONTESTED ELECTION Cornwall Council Election of Parish Councillors for Altarnun Parish Council on Thursday 6 May 2021 I, Kate Kennally, being the Returning Officer for the Parish of ALTARNUN PARISH COUNCIL at an Election of Parish Councillors for the said Parish report that the latest time for delivery of notices of withdrawal of candidature, namely Thursday 8 April 2021, having passed, the persons whose names appear in the accompanying list stood validly nominated and were duly elected without a contest. Name of Candidate Home Address Description (if any) BLOOMFIELD (address in Cornwall) Chris BRANCH 3 Penpont View, Five Lanes, Debra Ann Altarnun, Launceston, Cornwall, PL15 7RY COLES 17 St Nonnas Close, Altarnun, Lauren Launceston, PL15 7RU DOWLER (address in Cornwall) Craig Nicholas GREEN The Dovecote, Tredoggett Farm, Carl Stuart Altarnun, Launceston, Cornwall, PL15 7SA HOSKIN The Bungalow, Trewint Marsh, Tom Launceston, Cornwall, PL15 7TF KENDALL (address in Cornwall) Jason John MARSH 1 Todda Close, Bolventor, PL15 Health And Social Care Managing Leah Michelle 7FP Director SMITH (address in Cornwall) Polly Jane SMITH (address in Cornwall) Wesley Arthur Dated Tuesday 13 April 2021 Kate Kennally Returning Officer Printed and published by the Returning Officer, 3rd Floor, South Wing, County Hall, Treyew Road, Truro, TR1 3AY RETURN OF RESULT OF UNCONTESTED ELECTION Cornwall Council Election of Parish Councillors for Antony Parish Council on Thursday 6 May 2021 I, Kate Kennally, being the Returning Officer for the Parish of ANTONY PARISH COUNCIL at an Election of Parish Councillors for the said Parish report that the latest time for delivery of notices of withdrawal of candidature, namely Thursday 8 April 2021, having passed, the persons whose names appear in the accompanying list stood validly nominated and were duly elected without a contest. -

Notice of Election Election of Town and Parish Councillors Notice Is Hereby Given That 1
Notice of Election Election of Town and Parish Councillors Notice is hereby given that 1. Elections are to be held of Town and Parish Councillors for each of the under-mentioned Town and Parish Councils. If the elections are contested the poll will take place on Thursday 6 May, 2021. 2. I have appointed Holly Gamble, Claire Jenkin, Ruth Naylor, Sharon Richards, John Simmons, Geofrey Waxman and Alison Webb whose ofices are Room 11, Cornwall Council, St Austell Information Service, 39 Penwinnick Road, St Austell, PL25 5DR and 3S, County Hall, Truro TR1 3AY to be my Deputies and are specifically responsible for the following Towns and Parishes: Towns and Parishes within St Austell & Newquay Electoral Divisions (SAN) Seats Seats Seats Seats Carlyon (Carlyon Bay Ward) 7 Newquay (Whipsiderry Ward) 3 St Austell Bay (Porthpean & Trenarren Ward) 1 St Stephen-in-Brannel (Whitemoor Ward) 1 Carlyon (Tregrehan Ward) 2 Pentewan Valley (Pentewan Village Ward) 2 St Blaise (St Blaise North Ward) 6 Treverbyn (Bugle Ward) 4 Colan 11 Pentewan Valley (Tregorrick & Trewhiddle Ward) 7 St Blaise (St Blaise South Ward) 4 Treverbyn (Penwithick Ward) 11 Fowey 12 Roche 11 St Dennis 11 Tywardreath & Par (Highway Ward) 2 Grampound with Creed 9 St Austell (Bethel & Holmbush Ward) 7 St Enoder 14 Tywardreath & Par (Par Ward) 3 Mevagissey 14 St Austell (Central & Gover Ward) 7 St Ewe 10 Tywardreath & Par (Priory Ward) 5 Newquay (Central & Pentire Ward) 6 St Austell (Poltair & Mount Charles Ward) 6 St Mewan 13 Newquay (Porth & Tretherras Ward) 5 St Austell Bay (Charlestown