A Systematic Review of Therapeutic Approaches Used in Experimental Models of Interstitial Cystitis/Bladder Pain Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis
Review Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis Claudia Manini 1, Javier C. Angulo 2,3 and José I. López 4,* 1 Department of Pathology, San Giovanni Bosco Hospital, 10154 Turin, Italy; [email protected] 2 Clinical Department, Faculty of Medical Sciences, European University of Madrid, 28907 Getafe, Spain; [email protected] 3 Department of Urology, University Hospital of Getafe, 28905 Getafe, Spain 4 Department of Pathology, Cruces University Hospital, Biocruces-Bizkaia Health Research Institute, 48903 Barakaldo, Spain * Correspondence: [email protected]; Tel.: +34-94-600-6084 Received: 17 December 2020; Accepted: 18 February 2021; Published: 25 February 2021 Abstract: A broad spectrum of lesions, including hyperplastic, metaplastic, inflammatory, infectious, and reactive, may mimic cancer all along the urinary tract. This narrative collects most of them from a clinical and pathologic perspective, offering urologists and general pathologists their most salient definitory features. Together with classical, well-known, entities such as urothelial papillomas (conventional (UP) and inverted (IUP)), nephrogenic adenoma (NA), polypoid cystitis (PC), fibroepithelial polyp (FP), prostatic-type polyp (PP), verumontanum cyst (VC), xanthogranulomatous inflammation (XI), reactive changes secondary to BCG instillations (BCGitis), schistosomiasis (SC), keratinizing desquamative squamous metaplasia (KSM), post-radiation changes (PRC), vaginal-type metaplasia (VM), endocervicosis (EC)/endometriosis (EM) (müllerianosis), -

A New Look at the Etiology of Interstitial Cystitis/Bladder Pain Syndrome: Extraordinary Cultivations
International Urology and Nephrology (2019) 51:1961–1967 https://doi.org/10.1007/s11255-019-02248-5 UROLOGY - ORIGINAL PAPER A new look at the etiology of interstitial cystitis/bladder pain syndrome: extraordinary cultivations Tahsin Batuhan Aydogan1 · Oznur Gurpinar2 · Ozgen Koseoglu Eser2 · Begum Aydogan Mathyk3 · Ali Ergen1 Received: 25 April 2019 / Accepted: 24 July 2019 / Published online: 30 July 2019 © Springer Nature B.V. 2019 Abstract Purpose So far, studies have not clearly identifed infectious agents as an etiological factor for interstitial cystitis (IC). Spe- cifc microbiological diagnosis for detecting the pathogen with higher sensitivity in IC may decrease the treatment costs and increase psychosocial health of the patients. Methods A prospective clinical study was performed in 26 IC patients and 20 controls between April and September 2017. All participants were asked to give mid-stream urine sample for routine urine cultures. Followed by the negative results, symptomatic 26 patients were evaluated for L-form pathogen existence by extraordinary cultivation methods. Biopsy sam- ples were taken from 19 patients with ulcerative lesions in the bladder while collecting sterile urine samples from all 26 patients. PG broth, 5% sheep blood agar, EMB, Sabouraud’s dextrose, LEM, and GYPA were used. Followed by the 1st day inoculations, all inoculated PG broths were subcultured into the same solid media at the 2nd and 10th days in case of any growth after incubation of 24 h under 35–37 °C. The “O’Leary Sant Symptom and Problem Index” score forms were used to evaluate response to the appropriate treatment for those patients with documented pathogens. -

Interstitial Cystitis/Painful Bladder Syndrome
What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse What I need to know about Interstitial Cystitis/Painful Bladder Syndrome U.S. Department of Health and Human Services National Kidney and Urologic Diseases NATIONAL INSTITUTES OF HEALTH Information Clearinghouse Contents What is interstitial cystitis/painful bladder syndrome (IC/PBS)? ............................................... 1 What are the signs of a bladder problem? ............ 2 What causes bladder problems? ............................ 3 Who gets IC/PBS? ................................................... 4 What tests will my doctor use for diagnosis of IC/PBS? ............................................................... 5 What treatments can help IC/PBS? ....................... 7 Points to Remember ............................................. 14 Hope through Research........................................ 15 Pronunciation Guide ............................................. 16 For More Information .......................................... 17 Acknowledgments ................................................. 18 What is interstitial cystitis/painful bladder syndrome (IC/PBS)? Interstitial cystitis*/painful bladder syndrome (IC/PBS) is one of several conditions that causes bladder pain and a need to urinate frequently and urgently. Some doctors have started using the term bladder pain syndrome (BPS) to describe this condition. Your bladder is a balloon-shaped organ where your body holds urine. When you have a bladder problem, you may notice certain signs or symptoms. *See page 16 for tips on how to say the words in bold type. 1 What are the signs of a bladder problem? Signs of bladder problems include ● Urgency. The feeling that you need to go right now! Urgency is normal if you haven’t been near a bathroom for a few hours or if you have been drinking a lot of fluids. -

Pediatric Hemorrhagic Cystitis
CUA BEST PRACTICE REPORT Canadian Urological Association Best Practice Report: Pediatric hemorrhagic cystitis Jessica H. Hannick, MD, MSc1,2; Martin A. Koyle, MD, MSc2,3 1Division of Pediatric Urology, UH Rainbow Babies and Children’s Hospital, Cleveland, OH, United States; 2The Hospital for Sick Children, Toronto, ON, Canada; 3Division of Urology, Department of Surgery, University of Toronto, Toronto, ON, Canada Cite as: Can Urol Assoc J 2019;13(11):E325-34. http://dx.doi.org/10.5489/cuaj.5993 tem for guideline recommendations, as employed by the International Consultation on Urologic Disease (ICUD). Published online March 29, 2019 Definition HC is defined by the presence of hematuria and lower uri- Introduction nary tract symptoms, such as dysuria, frequency, or urgen- cy, in the absence of other potential contributing factors, This best practice report aims to provide the general practic- such as vaginal bleeding or bacterial or fungal urinary tract ing urologist with basic background information regarding infections.1 Multiple grading criteria have been published the pathophysiology and natural history of hemorrhagic cys- to distinguish the varied presentations of HC. Frequently titis (HC) in the pediatric population, as well as diagnostic referenced grading schema are Droller and Arthur’s, which and algorithmic therapeutic recommendations. Given that are used to aid the clinician in discerning potential treatment HC in the pediatric population is most frequently diagnosed options and inform the clinician about prognosis (Table 1).2,3 in the setting of bone marrow (BMT) or stem cell transplan- The European Organization for Research and Treatment of tation (SCT), discussion and recommendations will focus Cancer has combined similar grading criteria, along with largely on this population, but many of the diagnostic and quality of life parameters to further relay the morbidity and treatment options can be expanded to broader pediatric mortality implications of each grade.4 populations affected by HC. -
Symptoms of Overactive Bladder and Interstitial Cystitis – How Different?
183 Homma Y1, Tanaka M1, Niimi A1 1. Japan Red Cross Medical Centre SYMPTOMS OF OVERACTIVE BLADDER AND INTERSTITIAL CYSTITIS – HOW DIFFERENT? Hypothesis / aims of study Overactive bladder (OAB) is a symptom syndrome of urgency, frequency or urgency incontinence [1]. Interstitial cystitis (IC) is a bladder disease with a symptom syndrome of urgency, frequency, or bladder pain. Difference in symptoms of these confusable disorders is to be examined. Study design, materials and methods Female patients with OAB or IC and asymptomatic controls were recruited for the study. Diagnosis of OAB is based on 1) OAB symptom score [2] larger than 5 and/or urgency incontinence, and 2) exclusion of stress urinary incontinence and other obvious diseases. IC was diagnosed by 1) O’Leary & Sant’s symptom score [3] larger than 7 and/or bladder pain, 2) exclusion of other obvious diseases, and 3) Hunner’s ulcer or bladder bleeding at hydrodistension under anesthesia. The subjects recorded, in 3-day bladder diary, time voided, voided volume and intensity (0: none, 1: slightly, 2: moderately, 3: a lot) of 6 sensations or symptoms (urge to void, fear of leakage, amount of leakage, bladder discomfort, bladder pain and feeling of incomplete emptying) for each micturition. Additionally the urge questionnaire of 5 questions addressing the conditions during the last week was administered: 1) Did you feel urge to void that was not followed by voiding? (Response: yes, no), 2) Did you feel strong urge to void? (yes, no), 3) In case of yes in question 2, did you feel strong urge to void suddenly? (yes, no), 4) What happened or might happen, if you held strong urge to void for a long time, 1 hour for example ? (Response: leak urine, feel discomfort and/or pain, both), 5) In case of discomfort and/or pain in question 4, could you tell these 2 sensations apart? (yes, no). -

149 Treatment with Instillation of Hyaluronic
149 Collado Serra A1, Lopez-Guerrero J A2, Dominguez-Escrig J2, Ramirez Backhaus M2, Gomez-Ferrez A2, Mir C3, Casanova J3, Iborra I3, Casaña M3, Solsona E3, Arribas L3, Rubio-Briones J3 1. Fundación IVO. Valencia.Spain, 2. Fundación IVO, Valencia, Spain, 3. Fundación IVO, Valencia,Spain. TREATMENT WITH INSTILLATION OF HYALURONIC ACID IN PATIENTS WITH A HISTORY OF PELVIC RADIATION THERAPY AND URINARY STORAGE SYMPTOMS Hypothesis / aims of study Pelvic radiotherapy for the treatment of tumours located in the pelvis is not free of the risk of secondary irradiation of the bladder, producing a certain histological changes and it can result in both acute and chronic bladder injuries. Of these, the most evident and studied is radiation-induced hemorrhagic cystitis, although other urinary symptoms like frequency, urgency, incontinence, dysuria or pelvic pain has been described. Therefore, the objective of our paper is to evaluate the clinical utility of bladder instillation of hyaluronic acid in patients with a history of pelvic radiation therapy and storage symptoms with failure of previous treatment with anticholinergic Study design, materials and methods We considered 39 consecutive patients with storage urinary symptoms (defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence) due to post radiation cystitis treated with bladder instillation of hyaluronic acid. Severe urgency episodes with or without incontinence was measured using the PPIUS (Patient Perception of Intensity of Urgency Scale). Eligible patients had ≥three severe urgency episodes with or without incontinence during the 3-day voiding diary period, defined as PPIUS grades 3 and 4, and ≥eight micturitions/24 hours (1). -
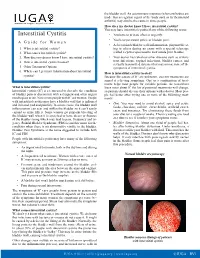
Interstitial Cystitis? You May Have Interstitial Cystitis If Any of the Following Occur: Interstitial Cystitis • You Have to Urinate Often Or Urgently
the bladder wall. An autoimmune response (when antibodies are made that act against a part of the body such as in rheumatoid arthritis) may also be the cause in some people. How does my doctor know I have interstitial cystitis? You may have interstitial cystitis if any of the following occur: Interstitial Cystitis • You have to urinate often or urgently. • You have persistent pelvic or bladder pain. A Guide for Women • A doctor finds bladder wall inflammation, pinpoint bleed- 1. What is interstitial cystitis? ing or ulcers during an exam with a special telescope 2. What causes interstitial cystitis? (called a cystoscope) used to look inside your bladder. 3. How does my doctor know I have interstitial cystitis? • Your doctor has ruled out other diseases such as urinary 4. How is interstitial cystitis treated? tract infections, vaginal infections, bladder cancer, and sexually transmitted diseases that may mimic some of the 5. Other Treatment Options symptoms of interstitial cystitis. 6. Where can I get more information about interstitial How is interstitial cystitis treated? cystitis? Because the causes of IC are unknown, current treatments are aimed at relieving symptoms. One or a combination of treat- ments helps most people for variable periods. As researchers What is interstitial cystitis? learn more about IC the list of potential treatments will change, Interstitial cystitis (IC) is a term used to describe the condition so patients should discuss their options with a doctor. Most peo- of bladder pain or discomfort with a frequent and often urgent ple feel better after trying one or more of the following treat- need to pass urine. -

Hemorrhagic Cystitis with Giant Cells in Rheumatoid Arthritis Treating with Tacrolimus
Journal of Rheumatic Diseases Vol. 21, No. 6, December, 2014 □ Case Report □ http://dx.doi.org/10.4078/jrd.2014.21.6.336 Hemorrhagic Cystitis with Giant Cells in Rheumatoid Arthritis Treating with Tacrolimus In Suk Min, YeonMi Ju, Hyun-young Kim, Yun Jung Choi, Won-Seok Lee, Wan-Hee Yoo Department of Rheumatology, Chonbuk National University Medical School and Research Institute of Clinical Medicine of Chonbuk National University Hospital-Chonbuk National University, Jeonju, Korea Hemorrhagic cystitis is a diffuse inflammation of the mu- hemorrhagic cystitis due to tacrolimus for the treatment cosa of the bladder, characterized by hematuria and burn- of rheumatoid arthritis. We describe a case of hemor- ing upon urination. This might be caused by a variety of rhagic cystitis with giant cells in a patient with rheumatoid reasons, including undergoing chemotherapy (such as cy- arthritis treating with tacrolimus. Hematuria resolved clophosphamide), radiation therapy, bladder cancer, cer- spontaneously with discontinuation of the drug. tain viruses, urinary infections, and thrombocytopenia. Key Words. Hemorrhagic cystitis, Rheumatoid arthritis, There are no previous reports of hemorrhagic cystitis asso- Tacrolimus ciated with the use of tacrolimus. This is the first case of Introduction with an occasional small clot. She was initially treated, em- Hemorrhagic cystitis (HC) is a diffuse inflammation of the pirically, with sulfamethoxazole/trimethoprim for a presumed mucosa of the bladder. It is characterized by gross hematuria urinary tract infection, but showed no change in symptoms. and irritating voiding symptoms such as dysuria, with fre- She was referred for further urologic evaluation. The patient quency and urgency (1). The reasons behind this may include denied any prior urologic history, and did not have a history undergoing chemotherapy (such as cyclophosphamide), using of smoking. -

Native Kidney Cytomegalovirus Nephritis and Cytomegalovirus Prostatitis in a Kidney Transplant Recipient
Received: 23 July 2018 | Revised: 20 August 2018 | Accepted: 2 September 2018 DOI: 10.1111/tid.12998 CASE REPORT Native kidney cytomegalovirus nephritis and cytomegalovirus prostatitis in a kidney transplant recipient Susanna K. Tan1 | Xingxing S. Cheng2 | Chia‐Sui Kao3 | Jenna Weber3 | Benjamin A. Pinsky1,3 | Harcharan S. Gill4 | Stephan Busque5 | Aruna K. Subramanian1 | Jane C. Tan2 1Department of Medicine, Division of Infectious Diseases and Geographic Abstract Medicine, Stanford University School of We present a case of cytomegalovirus (CMV) native kidney nephritis and prostatitis Medicine, Stanford, California in a CMV D+/R‐ kidney transplant recipient who had completed six months of CMV 2Department of Medicine, Division of Nephrology, Stanford University School of prophylaxis four weeks prior to the diagnosis of genitourinary CMV disease. The Medicine, Stanford, California patient had a history of benign prostatic hypertrophy and urinary retention that re‐ 3Department of Pathology, Stanford quired self‐catheterization to relieve high post‐voiding residual volumes. At 7 months University School of Medicine, Stanford, California post‐transplant, he was found to have a urinary tract infection, moderate hydrone‐ 4Department of Urology, Stanford University phrosis of the transplanted kidney, and severe hydroureteronephrosis of the native School of Medicine, Stanford, California left kidney and ureter, and underwent native left nephrectomy and transurethral re‐ 5Department of Surgery, Division of Abdominal Transplantation, Stanford section of the prostate. Histopathologic examination of kidney and prostate tissue University School of Medicine, Stanford, revealed CMV inclusions consistent with invasive CMV disease. This case highlights California that CMV may extend beyond the kidney allograft to involve other parts of the geni‐ Correspondence tourinary tract, including the native kidneys and prostate. -

Lesions of the Female Urethra: a Review
Please do not remove this page Lesions of the Female Urethra: a Review Heller, Debra https://scholarship.libraries.rutgers.edu/discovery/delivery/01RUT_INST:ResearchRepository/12643401980004646?l#13643527750004646 Heller, D. (2015). Lesions of the Female Urethra: a Review. In Journal of Gynecologic Surgery (Vol. 31, Issue 4, pp. 189–197). Rutgers University. https://doi.org/10.7282/T3DB8439 This work is protected by copyright. You are free to use this resource, with proper attribution, for research and educational purposes. Other uses, such as reproduction or publication, may require the permission of the copyright holder. Downloaded On 2021/09/29 23:15:18 -0400 Heller DS Lesions of the Female Urethra: a Review Debra S. Heller, MD From the Department of Pathology & Laboratory Medicine, Rutgers-New Jersey Medical School, Newark, NJ Address Correspondence to: Debra S. Heller, MD Dept of Pathology-UH/E158 Rutgers-New Jersey Medical School 185 South Orange Ave Newark, NJ, 07103 Tel 973-972-0751 Fax 973-972-5724 [email protected] There are no conflicts of interest. The entire manuscript was conceived of and written by the author. Word count 3754 1 Heller DS Precis: Lesions of the female urethra are reviewed. Key words: Female, urethral neoplasms, urethral lesions 2 Heller DS Abstract: Objectives: The female urethra may become involved by a variety of conditions, which may be challenging to providers who treat women. Mass-like urethral lesions need to be distinguished from other lesions arising from the anterior(ventral) vagina. Methods: A literature review was conducted. A Medline search was used, using the terms urethral neoplasms, urethral diseases, and female. -

Case Report Pseudomembranous Trigonitis in a Male with Klinefelter Syndrome: a Case Report and Evidence of a Hormonal Etiology
Int J Clin Exp Pathol 2014;7(6):3375-3379 www.ijcep.com /ISSN:1936-2625/IJCEP0000435 Case Report Pseudomembranous trigonitis in a male with Klinefelter syndrome: a case report and evidence of a hormonal etiology Derrick WQ Lian1, Fay X Li2, Caroline CP Ong2, CH Kuick1, Kenneth TE Chang1 Departments of 1Pathology and Laboratory Medicine, 2Paediatric Surgery, KK Women’s and Children’s Hospital, Singapore Received April 6, 2014; Accepted May 26, 2014; Epub May 15, 2014; Published June 1, 2014 Abstract: Klinefelter syndrome is a clinical syndrome with a distinct 47, XXY karyotype. Patients are characterized by a tall eunuchoid stature, small testes, hypergonotrophic hypogonadism, gynecomastia, learning difficulties and infertility. These patients have also been found to have raised estrogen levels. We report a 16 year old boy with Kline- felter syndrome presenting to our institution with gross hematuria. Cystoscopy and biopsy revealed the diagnosis of pseudomembranous trigonitis. Immunohistochemical stains showed an increase in estrogen and progesterone receptors in the trigone area but not in the rest of the bladder. In view of the patient’s mildly raised estrogen levels and the histological findings, we postulate that estrogen is the driver of the development of pseudomembranous trigonitis. This is the first reported case of pseudomembranous trigonitis seen in association with Klinefelter syn- drome, and also the first case of pseudomembranous trigonitis occurring within the male adolescent age group. Keywords: Klinefelter syndrome, pseudomembranous trigonitis, pediatric Introduction with a sense of incomplete voiding. These epi- sodes were initially treated with a course of oral Klinefelter syndrome is the most common chro- antibiotics by a primary care physician with no mosomal aberration in males and the most resolution of symptoms. -

Associated Diseases in Interstitial Cystitis/Bladder Pain Syndrome
Associated diseases in interstitial cystitis/bladder pain syndrome Joop P. van de Merwe [email protected] Associated diseases in IC/BPS patients Table 1. Examples of associated disorders diagnosed in IC/BPS patients in comparison with Associated diseases are diseases that occur more often the general population 2,5,6 together in the same person than by chance. Associated diseases for IC/BPS (interstitial cystitis/bladder pain diagnosis prevalence (%) syndrome) include allergy, fibromyalgia, irritable bowel IC/BPS general syndrome, Crohn's disease, ulcerative colitis, systemic lupus population erythematosus, rheumatoid arthritis and Sjögren's syndrome allergy 40.6 22.5 (table 1). irritable bowel syndrome 25.4 2.9 sensitive skin 22.6 10.6 Allergy vulvodynia 10.9 15.0 An allergy occurs when a person's immune system causes an fibromyalgia 12.8 3.2 adverse reaction to substances in the environment that are chronic fatigue syndrome 7.7 8.5 harmless to most other people. These substances (so-called migraine 18.8 18.0 allergens) are found in dust mites, pets, pollen, insects, ticks, asthma 9.2 6.1 15 moulds, foods and many medications. Adverse reactions Crohn’s disease/ulcerative colitis 7.3 0.07 without involvement of the immune system are called rheumatoid arthritis 4-13 1.0 intolerance. Intolerance mainly occurs to food ingredients and systemic lupus erythematosus 1.7 0.05 drugs. Sjögren’s syndrome 8.0 0.5 People experience different symptoms, depending on the allergen, the individual's immune system, previous contact with the same allergen and where it enters the body.