Old Plagues, New Threats: the Biotech Revolution and Its Impact on Us National Security
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biotechnology Research in an Age of Terrorism: Confronting the Dual Use Dilemma
Prepublication Copy BIOTECHNOLOGY RESEARCH IN AN AGE OF TERRORISM: CONFRONTING THE DUAL USE DILEMMA Committee on Research Standards and Practices to Prevent the Destructive Application of Biotechnology Development, Security, and Cooperation Policy and Global Affairs National Research Council OF THE NATIONAL ACADEMIES The National Academies Press Washington, D.C. www.nap.edu Prepublication Copy Uncorrected Proofs THE NATIONAL ACADEMIES 500 FIFTH STREET, N.W. Washington, D.C. 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the Committee responsible for the report were chosen for their special competences and with regard for appropriate balance. Financial Support: The development of this report was supported by the Alfred P. Sloan Foundation and the Nuclear Threat Initiative. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the organizations or agencies that provided support for the project. International Standard Book Number 0-309-09087-3 Additional copies of this report are available from National Academies Press, 2101 Constitution Avenue, N.W., Lockbox 285, Washington, D.C. 20055; (800) 624-6242 or (202) 334-3313 (in the Washington metropolitan area); Internet, //www.nap.edu Printed in the United States of America Copyright 2003 by the National Academy of Sciences. All rights reserved. Prepublication Copy Uncorrected Proofs The National Academy of Sciences is a private, nonprofit, self-perpetuating society of distinguished scholars engaged in scientific and engineering research, dedicated to the furtherance of science and technology and to their use for the general welfare. -

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

GAO-09-878R Project Bioshield Act: HHS Has Supported Development
United States Government Accountability Office Washington, DC 20548 July 24, 2009 Congressional Committees Subject: Project BioShield Act: HHS Has Supported Development, Procurement, and Emergency Use of Medical Countermeasures to Address Health Threats This report formally transmits the attached briefing in response to section 247d-6c of title 42 of the United States Code. (See the enclosure.) The statute required the Comptroller General to examine the Department of Health and Human Services’ (HHS) support for the development and procurement of and authority for the emergency use of medical countermeasures to address chemical, biological, radiological, and nuclear threats to public health, and provide the results to the congressional committees by July 21, 2009.1 HHS determines priorities for medical countermeasure procurement based on those chemical, biological, radiological, and nuclear agents that have been identified by the Department of Homeland Security as posing a material threat to the U.S. population that could affect national security. We provided the briefing to staff of your committees to satisfy the mandate reporting requirement on July 20, 2009, and July 21, 2009. – – – – – We are sending copies of this report to the Secretary of HHS, the Secretary of Homeland Security, and other interested parties. In addition, the report will be available at no charge on the GAO Web site at http://www.gao.gov. If you or your staff have any questions regarding this report, please contact me at (202) 512- 7114 or [email protected]. Contact points for our Offices of Congressional Relations and Public Affairs may be found on the last page of this report. -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

1540 Committee Matrix of Slovakia
1540 COMMITTEE MATRIX OF SLOVAKIA The information in the matrices originates primarily from national reports and is complemented by official government information, including that made available to inter-governmental organizations. The matrices are prepared under the direction of the 1540 Committee. The 1540 Committee intends to use the matrices as a reference tool for facilitating technical assistance and to enable the Committee to continue to enhance its dialogue with States on their implementation of Security Council Resolution 1540. The matrices are not a tool for measuring compliance of States in their non-proliferation obligations but for facilitating the implementation of Security Council Resolutions 1540 (2004), 1673 (2006), 1810 (2008) and 1977 (2011). They do not reflect or prejudice any ongoing discussions outside of the Committee, in the Security Council or any of its organs, of a State's compliance with its non-proliferation or any other obligations. Information on voluntary commitments is for reporting purpose only and does not constitute in any way a legal obligation arising from resolution 1540 or its successive resolutions. OP 1 and related matters from OP 5, OP 6, OP 8 (a), (b), (c) and OP 10 State: SLOVAKIA Date of Report: 2 November 2004 Date of First Addendum: 14 December 2004 Date of Second Addendum: 14 December 2007 Date of Committee Approval: Remarks (information refers Legally binding instruments, to the page of the organizations, codes of YES if YES, relevant information (i.e. signing, accession, ratification, -
![The Australia Group LIST of HUMAN and ANIMAL PATHOGENS and TOXINS for EXPORT CONTROL[1]](https://docslib.b-cdn.net/cover/3423/the-australia-group-list-of-human-and-animal-pathogens-and-toxins-for-export-control-1-443423.webp)
The Australia Group LIST of HUMAN and ANIMAL PATHOGENS and TOXINS for EXPORT CONTROL[1]
The Australia Group LIST OF HUMAN AND ANIMAL PATHOGENS AND TOXINS FOR EXPORT CONTROL[1] July 2017 Viruses 1. African horse sickness virus 2. African swine fever virus 3. Andes virus 4. Avian influenza virus[2] 5. Bluetongue virus 6. Chapare virus 7. Chikungunya virus 8. Choclo virus 9. Classical swine fever virus (Hog cholera virus) 10. Crimean-Congo hemorrhagic fever virus 11. Dobrava-Belgrade virus 12. Eastern equine encephalitis virus 13. Ebolavirus: all members of the Ebolavirus genus 14. Foot-and-mouth disease virus 15. Goatpox virus 16. Guanarito virus 17. Hantaan virus 18. Hendra virus (Equine morbillivirus) 19. Japanese encephalitis virus 20. Junin virus 21. Kyasanur Forest disease virus 22. Laguna Negra virus 23. Lassa virus 24. Louping ill virus 25. Lujo virus 26. Lumpy skin disease virus 27. Lymphocytic choriomeningitis virus 28. Machupo virus 29. Marburgvirus: all members of the Marburgvirus genus 30. Monkeypox virus 31. Murray Valley encephalitis virus 32. Newcastle disease virus 33. Nipah virus 34. Omsk hemorrhagic fever virus 35. Oropouche virus 36. Peste-des-petits-ruminants virus 37. Porcine Teschovirus 38. Powassan virus 39. Rabies virus and other members of the Lyssavirus genus 40. Reconstructed 1918 influenza virus 41. Rift Valley fever virus 42. Rinderpest virus 43. Rocio virus 44. Sabia virus 45. Seoul virus 46. Severe acute respiratory syndrome-related coronavirus (SARS-related coronavirus) 47. Sheeppox virus 48. Sin Nombre virus 49. St. Louis encephalitis virus 50. Suid herpesvirus 1 (Pseudorabies virus; Aujeszky's disease) 51. Swine vesicular disease virus 52. Tick-borne encephalitis virus (Far Eastern subtype) 53. Variola virus 54. -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor. -

Responding to the Threat of Agroterrorism: Specific Recommendations for the United States Department of Agriculture
Responding to the Threat of Agroterrorism: Specific Recommendations for the United States Department of Agriculture Anne Kohnen ESDP-2000-04 BCSIA-2000-29 October 2000 CITATION AND REPRODUCTION This document appears as Discussion Paper 2000-29 of the Belfer Center for Science and International Affairs and as contribution ESDP-2000-04 of the Executive Session on Domestic Preparedness, a joint project of the Belfer Center and the Taubman Center for State and Local Government. Comments are welcome and may be directed to the author in care of the Executive Session on Domestic Session. This paper may be cited as Anne Kohnen. “Responding to the Threat of Agroterrorism: Specific Recommendations for the United States Department of Agriculture.” BCSIA Discussion Paper 2000-29, ESDP Discussion Paper ESDP-2000-04, John F. Kennedy School of Government, Harvard University, October 2000. ABOUT THE AUTHOR Anne Kohnen graduated from the Kennedy School of Government, Harvard University, in June 2000, with a Master’s degree in public policy, specializing in science and technology policy. This paper is an extension of her Master’s thesis. ACKNOWLEDGEMENTS The author expresses special thanks go to the following people who contributed to this paper valuable information and expertise. From the USDA: Jerry Alanko, Dr. Bruce Carter, Dr. Tom Gomez, Dr. David Huxsoll, Dr. Steve Knight, Dr. Paul Kohnen, Dr. Marc Mattix, Dr. Norm Steele, Dr. Ian Stewart, Dr. Ty Vannieuwenhoven, Dr. Tom Walton, and Dr. Oliver Williams. From other agencies: Dr. Norm Schaad (USAMRIID), Dr. Tracee Treadwell (CDC). From the Kennedy School of Government: Dr. Richard Falkenrath, Greg Koblentz, Robyn Pangi, and Wendy Volkland. -
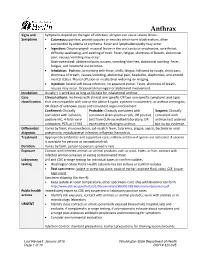
Anthrax Reporting and Investigation Guideline
Anthrax Signs and Symptoms depend on the type of infection; all types can cause severe illness: Symptoms • Cutaneous: painless, pruritic papules or vesicles which form black eschars, often surrounded by edema or erythema. Fever and lymphadenopathy may occur. • Ingestion: Oropharyngeal: mucosal lesion in the oral cavity or oropharynx, sore throat, difficulty swallowing, and swelling of neck. Fever, fatigue, shortness of breath, abdominal pain, nausea/vomiting may occur. Gastrointestinal: abdominal pain, nausea, vomiting/diarrhea, abdominal swelling. Fever, fatigue, and headache are common. • Inhalation: Biphasic, presenting with fever, chills, fatigue, followed by cough, chest pain, shortness of breath, nausea/vomiting, abdominal pain, headache, diaphoresis, and altered mental status. Pleural effusion or mediastinal widening on imaging. • Injection: Severe soft tissue infection; no apparent eschar. Fever, shortness of breath, nausea may occur. Occasional meningeal or abdominal involvement. Incubation Usually < 1 week but as long as 60 days for inhalational anthrax Case Clinical criteria: An illness with at least one specific OR two non-specific symptoms and signs classification that are compatible with one of the above 4 types, systemic involvement, or anthrax meningitis; OR death of unknown cause and consistent organ involvement Confirmed: Clinically Probable: Clinically consistent with Suspect: Clinically consistent with isolation, consistent Gram-positive rods, OR positive consistent with positive IHC, 4-fold rise in test from CLIA-accredited laboratory, OR anthrax test ordered antibodies, PCR, or LF MS epi evidence relating to anthrax but no epi evidence Differential Varies by form; mononucleosis, cat-scratch fever, tularemia, plague, sepsis, bacterial or viral diagnosis pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care; anthrax antitoxin if spores are activated. -

Bioterrorism & Biodefense
Hugh-Jones et al. J Bioterr Biodef 2011, S3 Bioterrorism & Biodefense http://dx.doi.org/10.4172/2157-2526.S3-001 Review Article Open Access The 2001 Attack Anthrax: Key Observations Martin E Hugh-Jones1*, Barbara Hatch Rosenberg2 and Stuart Jacobsen3 1Professor Emeritus, Louisiana State University; Anthrax Moderator, ProMED-mail, USA 2Sloan-Kettering Institute for Cancer Research and State Univ. of NY-Purchase (retired); Scientists Working Group on CBW, Center for Arms Control and Non-Proliferation, USA 3Technical Consultant Silicon Materials, Dallas, TX,USA Abstract Unresolved scientificquestions, remaining ten years after the anthrax attacks, three years after the FBI accused a dead man of perpetrating the 2001 anthrax attacks singlehandedly, and more than a year since they closed the case without further investigation, indictment or trial, are perpetuating serious concerns that the FBI may have accused the wrong person of carrying out the anthrax attacks. The FBI has not produced concrete evidence on key questions: • Where and how were the anthrax spores in the attack letters prepared? There is no material evidence of where the attack anthrax was made, and no direct evidence that any specific individual made the anthrax, or mailed it. On the basis of a number` of assumptions, the FBI has not scrutinized the most likely laboratories. • How and why did the spore powders acquire the high levels of silicon and tin found in them? The FBI has repeatedly insisted that the powders in the letters contained no additives, but they also claim that they have not been able to reproduce the high silicon content in the powders, and there has been little public mention of the extraordinary presence of tin. -

Chapter 4 Considering US Proposals for Enhanced Biosafety, Biosecurity, and Research Oversight
Chapter 4 Considering US Proposals for Enhanced Biosafety, Biosecurity, and Research Oversight f the proposals that the US government tabled to stand in for a monitoring protocol for the OBiological and Toxin Weapons Convention (BWC), the industry experts reacted most positively to those calling for BWC members to improve standards for biosafety, biosecurity, and oversight of genetic engineering research. They tempered their praise with criticism of the state-by-state structure of the proposals, which they saw as undercutting their potential efficacy. The industry group recommended instead that the United States advocate international adoption of common minimum standards in each of the areas, based on current US regulations and guidelines, or their equivalent. Taking the US government’s biosafety, biosecurity, and research oversight initiatives in turn, this chapter reviews the industry group’s discussion of pertinent domestic efforts. The industry experts then explain why some changes are needed domestically and how to achieve them. Improvements at home will pave the way for the successful promotion of similar initiatives internationally. As one participant noted, “If we don't walk before we run, if we don't set up things at home first to serve as an international model, I'm not going to count on anyone else to do it.”1 In each topical area, the industry group makes specific recommendations to strengthen the US proposals. Prior to the discussion of the individual proposals, the discussion briefly focuses on three factors that will underpin the viability of any international moves to enhance biosecurity, biosafety, and oversight of genetic engineering research. The first factor key to the success of any new standards will be the articulation of agreed lists of select human, animal, and plant pathogens. -

Project Bioshield Act of 2004
PUBLIC LAW 108–276—JULY 21, 2004 118 STAT. 835 Public Law 108–276 108th Congress An Act To amend the Public Health Service Act to provide protections and countermeasures against chemical, radiological, or nuclear agents that may be used in a terrorist attack against the United States by giving the National Institutes of Health July 21, 2004 contracting flexibility, infrastructure improvements, and expediting the scientific [S. 15] peer review process, and streamlining the Food and Drug Administration approval process of countermeasures. Be it enacted by the Senate and House of Representatives of the United States of America in Congress assembled, Project BioShield Act of 2004. SECTION 1. SHORT TITLE. 42 USC 201 note. This Act may be cited as the ‘‘Project BioShield Act of 2004’’. SEC. 2. BIOMEDICAL COUNTERMEASURE RESEARCH AND DEVELOP- MENT—AUTHORITIES. (a) IN GENERAL.—Part B of title III of the Public Health Service Act (42 U.S.C. 243 et seq.) is amended by inserting after section 319F the following section: ‘‘SEC. 319F–1. AUTHORITY FOR USE OF CERTAIN PROCEDURES 42 USC 247d–6a. REGARDING QUALIFIED COUNTERMEASURE RESEARCH AND DEVELOPMENT ACTIVITIES. ‘‘(a) IN GENERAL.— ‘‘(1) AUTHORITY.—In conducting and supporting research and development activities regarding countermeasures under section 319F(h), the Secretary may conduct and support such activities in accordance with this section and, in consultation with the Director of the National Institutes of Health, as part of the program under section 446, if the activities concern qualified countermeasures. ‘‘(2) QUALIFIED COUNTERMEASURE.—For purposes of this section, the term ‘qualified countermeasure’ means a drug (as that term is defined by section 201(g)(1) of the Federal Food, Drug, and Cosmetic Act (21 U.S.C.