Effectiveness of Motivational Interviewing in Improving Lipid Level
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Itallicios OREES, Mos 1'90 Y Di 11Dmihisideicill DE Pisticill Kilogramo De Carbón 1'87 Ldem De Letia 0,37 *FERNAN-NUÑEZ
• 1£757 Nú m. 289 Viernes 17 de diciembre de 1954 ICONCERTADO ADVERTENCIAS PESETAS PESETAS Número suelto clei ato acuna... • VCOsitas.. Precios suscripción Capital Provincias Los Alcaldes y Secretarios dispondrán se fije un ejemplar del B. O. en el sitio público Número suelto del ato anterior ... 2'00 Trimestre 36 45 de costumbre y permanecerá hasta que reciban Número suelto de2allos anteriores 3'00 • 66 84 el siguiente.—Toda clase de anuncios se envia- Semestre rán directamente al Excmo. Sr. Gobernador Número suelto de loe ellos anterie Año 120 130 Civil para que autorice su inserción. res a les dos últimos • e00 • 3 ptas. Linea o parte de ella, No se publica los Domingos ni dies festivos. SUMARIO •• Pägtn.I • ANUNCIOS OFICIALES, PARTICULM ES i • i'418$1.!a T DE ADMINISTRACIÓN DE JUSTICIA • . Ayuntamientos, — Fernán-Niiiieg, Patina del Río, Vi- de p^e- . Diputación Provincial de Córdota.—Nota dios de los artículos suministrados a fuerzas del Eid.r- lle harta, Benamejf; Bujalance, Baena, lenájar, Nueva Zito y Guardia Civil durante el pasado mes de noviem- Carteya, Almedinilla, Palenciana. San Sebastián de los bre por los pueblos de esta provincia 1.757 Ballesteros y Pozoblanco . , Juntos Municipales del Censo Electotal.—El Guijo 1.757 Juzgados.— Rute Baena, Montoro, Cabra y Motril. 1.760- ^ kiem de paja de 6 kilogra- AYL1 N TA N'O E 1n4 T;15:2•g, itallicios OREES, mos 1'90 Y Di 11DMIHISIDEICIll DE PISTICIll Kilogramo de carbón 1'87 ldem de letia 0,37 *FERNAN-NUÑEZ. Litro de petróleo 3'03 Núm. 4.770 Diputación Provincial [dem de aceite 1268 Don Pedro Laguna CresitO,7Al.1 de Córdoba Lo que se hace público para calde Presidente del 'Ay*IMIL+2 general conocimiento de los Ayun- • miermi de eta viIJ de Ñrrosan•- Núm. -

BOLETIN OFICIAL DEL ESTADO Subasta La Adjudicación Dt Las Obras Que Se Mencionan I
4.223 ^ BOLETIN OFIC IAL DELAPROVINCIA DE CURDOBA i FRANQUEO ^^ R 1\Ú111. '^;^(i I. ^L1E'rCtlll!S, J3 de OCtlll)rl' '1S)fir) Ii DEPÓSITO LECiAL, CO. 1.-1958 I CONCERTADO IL .^ ^ - _ _ ^ ADVERTÉNCL9.-Los Alcaldes y Secretarios dispondrán se TARIFA DE SUSCRIPCION fije un ejemplar del B. O. en el sítio público de costumbre y permanecerá hasta que reciban el siguiente.-Toda claae Trime^tr Año Semestre de anuncios se enviarán directamente al Excmo. Sr. (^o- bernador Civil para que autorice su inserción. -. : ?a Capital . 550'00 375'00 200'00 NO SE PUBLICA DOMINC30S NI DIAS FESTIVOS • - "_.: ^:.arov?ncla y otros puntos . 600'00 450'00 250'00 EDICTOS DE PAC30: Línea o parte de ella, NUEVE pesetas ^'enta de ejemplares: Del aí^o actual, 3 pts.; de dos aSos anteriores, 6 pta., y anteriores a los años indicadbs, 10 pta. -------- S U M A R I O _ _-- ---------- PAGINA PACINA ADMINISTRACION ECONOMICA Recaudaclón de Contríbuciones. Zona de Pozo- úOBIER,NO CIVIL • blanco. - i:xpcdiente por débitos de Tasas Sanitarias ... 1.227 fielación de licencias dr raia cxpedidas durante cl mes de j^^nio de 19fi5. (Continualú) •. ••-- • 1 223 ANUNCIOS DE SUBASTA Miniaterio de Educación Nacional. - Anunciando a BOLETIN OFICIAL DEL ESTADO subasta la adjudicación dt las obras que se mencionan I. Dispoxiciones generales. Ministerio de la Go• en el Instituto Nacional de Enseñanza Media en Pozo• bernación. - Orden por la que se aprueban las Instruc- blanco (Córdoba) . ...... ...... ......... .. 1.227 ciones para la formacirín y tramitación de los presupues- Delegación de Hacienda de Córdoba.-Subasta de tos de los Corporaciones Locales. -

Sierra Morena De Córdoba Morena Sierra 23 1
Sierra Morena de Córdoba Morena Sierra 23 1. Identificación y localización La Sierra Morena cordobesa es un territorio serrano con Los pueblos se integran adecuadamente en el paisaje, paisajes naturales muy antropizados para actividades de con sus ruedos, caseríos tradicionales e hitos religiosos agrosilvicultura, sobre todo ganaderas y forestales. La que dan jerarquía a la mirada sobre ellos. No existe una dehesa es el elemento paisajístico más significativo y tal capital de todo el ámbito, si bien hay numerosas pobla- vez el mejor ejemplo en el territorio andaluz del aprove- ciones que ejercen el papel de cabeza comarcal (Villanue- chamiento y uso sostenible de los recursos naturales. Las va del Rey, Villaviciosa de Córdoba, Fuente Obejuna). La sierras atraviesan la provincia de este a oeste, separando minería también ha dado centralidad y dejado paisajes la vega de Los Pedroches en un la zona oriental y cen- de gran interés en Peñarroya-Pueblonuevo. tral y prolongándose hacia el noroeste en el valle del alto Guadiato. Las formas suaves y acolinadas conforman el Esta demarcación se encuadra dentro de las áreas pai- paisaje del bosque aclarado y explotado de la encina y el sajísticas de Sierras de baja montaña y Campiñas de lla- alcornoque. nuras interiores. Reseñas patrimoniales en el Plan de Ordenación del Territorio de Andalucía (pota) Zonificación del POTA: valle del Guadiato-Los Pedroches, vega del Guadalquivir, centro regional de Córdoba y Montoro (dominio territorial del valle del Guadalquivir) Referentes territoriales para la -

[email protected] [email protected]
MUNICIPIO CARGO NOMBRE E_MAIL DIRECCIÓN CP WEB Técnico http://www.adamuz.es/ Adamuz José Mª Marín Pérez [email protected] C/ De la Fuente, nº 1 14.430 Deportivo Aguilar de la Técnico de Plaza de San José, nº http://www.aguilardelafrontera.es Miguel Pavón Reina [email protected] 14.920 Frontera Deportes 1 / Técnico de Plaza de la Alcaracejos Sergio Álvarez Simancas [email protected] 14.480 http://www.alcaracejos.es/ Deportes Constitución, 1 Técnico de Almedinilla Oscar Ruiz [email protected] Plaza de España, 12 14812 http://www.almedinilla.es/ Deportes Almodovar Del Técnico de Plaza De La http://www.almodovardelrio.com Sergio Izquierdo Pérez [email protected] 14720 Río Deportes Constitucion, 4 / Técnico de Añora Sabino Luna Antolí [email protected] C/. Cerrillo, 4 14.450 http://www.anora.es/ Deportes Plaza De La Baena Gerente DMD Rafael Santano Cañete [email protected] 14850 http://www.baena.es/deportes Constitución, 1 Técnica de deportesbelalcazar_6@hotmail. Plaza de la http://deportesayuntamientodebe Belalcázar Cecilia Benitez Suarez 14.280 Deportes com Costitución, 11 lalcazar.blogspot.com.es/ Técnico de http://belmez.guadiato.dnsalias.n Belmez Mª Fernández Rama [email protected] Cordoba, 1 14240 Deportes et/ Técnico de Plaza de la Benamejí Juan Jesús Adalid Leiva [email protected] 14910 http://www.benameji.es/ Deportes Constitución, S/N Técnico http://www.bujalance.es/index.ph Bujalance Francisco S. Larrosa Lizarte [email protected] Plaza Mayor, nº 1 14.650 Deportivo p?page=deportes GERENTE Cabra Antonio Cazorla Campos [email protected] C/ Palomas, 2 14940 http://www.deportecabra.es/ PMD Cañete de las Técnico http://www.aytocanetedelastorres. -

Ated in Specific Areas of Spain and Measures to Control The
No L 352/ 112 Official Journal of the European Communities 31 . 12. 94 COMMISSION DECISION of 21 December 1994 derogating from prohibitions relating to African swine fever for certain areas in Spain and repealing Council Decision 89/21/EEC (94/887/EC) THE COMMISSION OF THE EUROPEAN COMMUNITIES, contamination or recontamination of pig holdings situ ated in specific areas of Spain and measures to control the movement of pigs and pigmeat from special areas ; like Having regard to the Treaty establishing the European wise it is necessary to recognize the measures put in place Community, by the Spanish authorities ; Having regard to Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra Community trade in bovine animals and swine (') as last Whereas it is the objective within the eradication amended by Directive 94/42/EC (2) ; and in particular programme adopted by Commission Decision 94/879/EC Article 9a thereof, of 21 December 1994 approving the programme for the eradication and surveillance of African swine fever presented by Spain and fixing the level of the Commu Having regard to Council Directive 72/461 /EEC of 12 nity financial contribution (9) to eliminate African swine December 1972 on animal health problems affecting fever from the remaining infected areas of Spain ; intra-Community trade in fresh meat (3) as last amended by Directive 92/ 1 18/EEC (4) and in particular Article 8a thereof, Whereas a semi-extensive pig husbandry system is used in certain parts of Spain and named 'montanera' ; whereas -

Of Council Regulation (EC)
13.5.2010 EN Official Journal of the European Union C 125/19 V (Announcements) OTHER ACTS EUROPEAN COMMISSION Publication of an application pursuant to Article 6(2) of Council Regulation (EC) No 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs (2010/C 125/10) This publication confers the right to object to the application pursuant to Article 7 of Council Regulation (EC) No 510/2006 ( 1 ). Statements of objection must reach the Commission within six months of the date of this publication. SINGLE DOCUMENT COUNCIL REGULATION (EC) No 510/2006 ‘MONTORO-ADAMUZ’ EC No: ES-PDO-0005-0658-08.11.2007 PGI ( ) PDO ( X ) 1. Name: ‘Montoro-Adamuz’ 2. Member State or Third Country: Spain 3. Description of the agricultural product or foodstuff: 3.1. Type of product: Class 1.5 Oils and fats (butter, margarine, oil, etc.) 3.2. Description of the product to which the name in (1) applies: Extra virgin olive oil obtained from the fruit of the olive tree (Olea Europaea L.), of the following varieties: Nevadillo Negro, Picual, Lechín de Sevilla, Picudo and Carrasqueño de la Sierra. The native variety Nevadillo Negro, when more than 10 %, and the Picual variety are considered the main varieties, both representing more than 98 % in the blend of oils. Extraction takes place exclusively by mechanical or physical processes that do not modify the oil, but preserve the flavour, fragrance and characteristics of the fruit from which it is obtained. The oils have the following physico-chemical characteristics: Acidity Maximum 0,5 % Humidity Maximum 0,1 % Peroxide value Maximum 20 mEq. -

History of a Guerilla Band: the Three Jubiles Brothers
The Anarchist Library (Mirror) Anti-Copyright History of a Guerilla Band: The three Jubiles brothers Antonio Téllez Solà January 2000 The three Jubiles brothers took to the hills in late March1939 and marauded through the hills around Villaviciosa, Almodóvar and Hornachuelos, before settling in the Montoro highlands. The Bujalance district of Córdoba province, where theCNT predominated, happened by a freak to escape the army’s Rising on 18 July 1936. In Bujalance the Civil Guard confined itself to staying in barracks and never lifted a finger, in spite of pres- sures from local rightists doubtless afraid of the power of the anarcho-syndicalist labour organisation. In the end, on 25 July, Antonio Téllez Solà the Civil Guard placed itself at the disposition of the Popular History of a Guerilla Band: The three Jubiles brothers Front. The garrison was shipped out to Jaén or to Madrid, ex- January 2000 cept for one sergeant and two Guards accused of having imple- Retrieved on 17th May 2021 from mented the ley de fugas (shooting ‘escaping’ prisoners) in the www.katesharpleylibrary.net Cañetejo ravine back in December 1933; these were executed Published in Polémica (Barcelona), no. 70, January 2000. in Cañetejo on 25 July. Translated by: Paul Sharkey. From the very outset, a Popular Front was established: it was made up of nine members, three of them from the CNT: usa.anarchistlibraries.net these were Francisco Garcia Cabello (aka El Niño del Aceite) who had been sentenced to death following the revolutionary events of December 1933, Bartolomé Parrodo Serrano and Ilde- fonso Coca Chocero (aka El Viejo). -
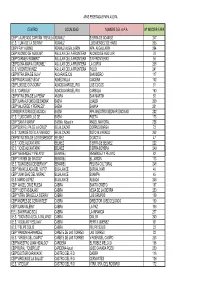
Centro Localidad Nombre Del Apa Nº
APAS FEDERADAS FAPA AGORA CENTRO LOCALIDAD NOMBRE DEL A.P.A. Nº INSCRIP.FAPA CEIP "LAUREADO CAPITÁN TREVILLA"ADAMUZ SIERRA DE ADAMUZ 247 I.E.S. "LUNA DE LA SIERRA" ADAMUZ LUIS MENDEZ DE HARO 206 CEIP FRAY ALBINO ADAMUZ-ALGALLARIN APA ALGALLARIN 294 CEIP"ALONSO DE AGUILAR" AGUILAR DE LA FRONTERA ALONSO DE AGUILAR 79 CEIP"CARMEN ROMERO" AGUILAR DE LA FRONTERA SIN FRONTERAS 56 CEIP"DOÑA MARIA CORONEL" AGUILAR DE LA FRONTERA LA CURVA 208 I.E.S. VICENTE NUÑEZ AGUILAR DE LA FRONTERA POLEY 241 CEIP"NTRA.SRA.DE GUIA" ALCARACEJOS SAN ISIDRO 117 CEIP"RODRIGUEZ VEGA" ALMEDINILLA CAICENA 102 CEIP"LUIS DE GONGORA" ALMODOVAR DEL RIO LOS CUCOS 91 I.E.S. "CARBULA" ALMODOVAR DEL RIO CARBULA 160 CEIP"NTRA.SRA.DE LA PEÑA" AÑORA SAN MARTIN 93 CEIP"JUAN ALFONSO DE BAENA" BAENA JUALBA 209 CEIP"VALVERDE Y PERALES" BAENA BAIANA 201 CONSERVATORIO DE MÚSICA BAENA APA MAESTRO BEDMAR ENCINAS 282 I.E.S. "LUIS CARRILLO DE BAENA POETA 173 CEIP "SANTA MARÍA" BAENA. Albendín ANGEL MAYORAL 258 CEIP"SOR FELIPA DE LA CRUZ" BELALCAZAR CORPUS BARGA 73 I.E.S. "JUAN DE SOTO ALVARADO" BELALCAZAR SOTO ALVARADO 262 CEIP"NTRA.SRA.DE LOS REMEDIOS" BELMEZ DIDACTIA 47 I.E.S. "JOSÉ ALCANTARA" BELMEZ SIERRA DE BELMEZ 222 I.E.S. "JOSÉ ALCANTARA" BELMEZ SIERRA BOYERA 249 CEIP "MENENDEZ Y PELAYO" BENAMEJÍ MENENDEZ Y PELAYO 82 CEIP "VIRGEN DE GRACIA" BENAMEJÍ EL JARDÍN 113 I.E.S. "DON DIEGO DE BERNUY" BENAMEJÍ PELUSA CULTURAL 248 CEIP "INMACULADA DEL VOTO" BUJALANCE BORJALIMAR 46 CEIP "JUAN DIAZ DEL MORAL" BUJALANCE BUAMPA 45 I.E.S. -

El Niño Mártir De Puente Genil Sara Baena
El niño mártir de Puente Genil Sara Baena Fernández A mi padre Aunque han pasado más de dos siglos, el acontecimiento que tuvo lugar en la Puente de don Gonzalo en 1731, sigue estremeciendo y moviendo a la compasión, pues nada hay más vil que el maltrato a un niño. Si lo pensamos, no es un hecho tan alejado en el tiempo; en la actualidad son conocidos los casos de bebes robados, secuestrados, asesinados por sus padres, un familiar o un desconocido, y debemos preguntarnos cuál es la motivación de esa manera de actuar y si llegaremos a justificarlos. Son frecuentes los casos de niños mártires repartidos por toda la geografía española, por ejemplo, el niño de la Guardia o de Sepúlveda. En ambos casos, se culpó a los judíos de su muerte, debido sobre todo a la animadversión y odio hacia esta minoría étnica durante la edad moderna. Las acusaciones fueron tan frecuentes en la España moderna que dieron lugar a algunos romances, como el de El judío de Toledo1. Sin embargo, no es éste el caso de la Puente de don Gonzalo, pues aunque el martirio tuvo lugar en la víspera del Día de los Santos Inocentes, en las fuentes no se han encontrado evidencias de que los culpables fueran supuestos judeoconversos. Debido a esto, el asesinato del Alonso no se encasilla, desde mi punto de vista en uno ritual, a pesar de las heridas que mostraba el pequeño. El protagonista de esta historia envuelta en la leyenda se llamaba Alonso Ruperto de los Ríos, nacido el 27 de marzo de 1728, y siendo su padrino de bautismo2 D. -

La Crónica Del Alto Guadalquivir
La Crónica del NOVEMBRE DEL 2020 Alto EJEMPLAR GRATUITO Nº 225 DIRECTOR: RAFAEL ROMERO CASTILLO DEPÓSITO LEGAL: CO-1335/2001 Guadalquivir LA JUNTA PRESUPUESTA PARA EL7 LA HERMANDAD DEL SANTO 10 IPRODECO DESTINA 12.000 11 2021 EL NUEVO CENTRO DE ENTIERRO DE PEDRO ABAD EUROS A LA DINAMIZACIÓN DEL SALUD DE MONTORO PRESENTA UNA NUEVA TALLA COMERCIO DE VILLAFRANCA CRÓNICA Un positivo entre 684 de cribados masivos La Junta de Andalucía ha realizado test en los municipios de Bujalance, Montoro y Villa del Río para detectar la situación de los contagios La Crónica de Alto Guadalquivir 2 Actualidad NOVIEMBRE DEL 2020 Análidad El Covid-19 en la comarca ; Solo un positivo en los cribados masivos hechos en Bujalance, Montoro y Villa del Río Entre los tres municipios fueron 684 las personas sometidas a los test, demostrando un descenso de la curva de contagios CRÓNICA R. CASTRO J. ESCAMILLA ALTO GUADALQUIVIR/ a comarca del Alto Guadalquivir ha sido pun- tera en la realización de cribados, debido al alto Líndice de contagios de Covid-19 producido en varios munici- pios. Si en octubre fue en El Carpio, en noviembre le ha toca- do a Bujalance, Montoro y Villa del Río. Tras el cribado realizado en Bujalance, no se registró nin- gún positivo de los 227 test rápi- dos de antígenos realizados por el Servicio Andaluz de Salud en el Pabellón Municipal de Deportes Pepe Montalbán, un cribado masivo que se ha reali- zado de forma voluntaria, ya que aunque actualmente Bujalance está bajando conside- rablemente en el número de afectados, llegó a tener una inci- dencia muy alta de coronavirus. -

Partido De Agnilar. Partido De Baena. Partido De Bulalanoe . Partido De
_ss — PROVINCIA DE CÓRDOB A Comprende esta provincia los siguientes ayuntamientos por partidos judiciales : Partido de Agnilar. Aguilar. 1 Monturque . Puente-Genil . Partido de Baena. Baena. 1 Luque. 1 Valenzuela . Partido de Bulalanoe . Bujalance. Cañete de las Torres . 1 Carpio (El). 1 Pedro Abad . Partido de Cabra. Cabra. Doña Mencía. Nueva-Carteya. Zuheros. Partido de Castro del Rio . Castro del Río. 1 Espejo. Partido de Córdoba. CÓRDOBA. ~ Ovejo. Villaviciosa. Partido de Fuenteovejuna . Bélmez. Fuenteovejuna. Pueblonuevo del Terri - Villaharta. ble. Blázquez. Granjuela (La) . Villanueva del Rey . Espiel. Peñarroya. Valsequillo . Partido de Hinojosa del Duque . Villaralto. Belalcázar. Hinojosa del Duque . Santa Eufemia. Fuente la Lancha. Viso (El). Partido de Lucena. Encinas Reales. 1 Lucena. Partido de Montilla. Montilla. Partido de Montoro. Adamuz. Montoro . 1 Villa del Río . 1 Villafinca de Córdoba . Córdoba Tomo I. Resultados definitivos. Detalle por provincias Fondo documental del Instituto Nacional de Estadística 1/4 Partido de Posadas . Alnvuióvar del Río . Fuente-Palmera . 1lc rnachuehís . Posadas . Carlota (I,a) . Guadalcazar . Palma del Río . Partido de Pozoblanco . Alcaracejos . Dos-"forres . Pedroche . Torrecampo . Añora. Villanueva de Córdoba . Conquista . Guijo. Pozohlanco. Villanueva del Duque . Partido de Priego de Córdoba . Almedinilla . 1 Carcahucy. Fuente-Cejar. Prie o de Córdoba . Partido de Zambia (La) . Fermín-Núñez . Nlonteuia y 1 San Sebastián de los Ra-1 Santaella . I lontalbán . Rambla (La J . llesteros . Victoria (La) . Partido de auto . Renamcjí . Iznáiar. Palee ciana. Rute. TOTAL DE = A PROVINCI A Partido~ judiciales Ayuntamientos 74. Córdoba Tomo I. Resultados definitivos. Detalle por provincias Fondo documental del Instituto Nacional de Estadística 2/4 t ' I::AS1 I H: 1. -

Campiña De Córdoba
Bienes, Paisajes e Itinerarios Campiña de Córdoba Esta sección ha sido elaborada, además de por los firmantes, gracias a la colaboración de Silvia Fernández Cacho, Valle Muñoz Cruz, Isabel Durán Salado, David Villalón Torres, Gema Carrera Díaz, Plácido González Martínez y Lorena Ortiz Lozano Quien observe la campiña cordobesa identificará el olivar como elemento protagonista de su paisaje de suaves lomas, pero un mayor acercamiento permite descubrir un mosaico de colores y texturas, y multitud de aprovechamientos. El olivo convive con el cultivo de cereales y otros de regadío que configuran un territorio marcado por su sistema productivo agrario y la industria asociada (elaboración de aceites, vinos, tonelería, etc.), y cuya riqueza se pone de manifiesto en el legado patrimonial y cultural que han dejado sus pobladores a lo largo de la historia: yacimientos, fortificaciones y castillos. La campiña de Córdoba, a diferencia de otras zonas interiores de Andalucía, ha resistido la tendencia general al debilitamiento: sus recursos naturales y su valor ambiental y paisajístico han permitido que sus ciudades mantengan las tasas de crecimiento, mejoren sus equipamientos y servicios, y constituyan, en definitiva, perspectivas de futuro sobre unas bases de desarrollo bien establecidas. Lagar La Primilla en Montilla. Foto: Juan Carlos Cazalla, IAPH Bienes, Paisajes e Itinerarios Bienes, Paisajes Revista ph • Instituto Andaluz del Patrimonio Histórico • nº 76 • noviembre 2010 • pp. 20-67 • 021 Campiña de Córdoba en Fernán Núñez. Foto: Juan Carlos Cazalla, IAPH Bienes, Paisajes e Itinerarios Bienes, Paisajes 022 • Revista ph • Instituto Andaluz del Patrimonio Histórico • nº 76 • noviembre 2010 • pp. 20-67 Bienes, Paisajes e Itinerarios Campiña de Córdoba La campiña cordobesa María D.