Reproductive Morphology and Genetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Productivity of Castor Oil Plant (Ricinus Communis L.) in the North of Sinaloa
Revista Mexicana Ciencias Agrícolas volume 10 number 5 June 30 - August 13, 2019 Article Productivity of castor oil plant (Ricinus communis L.) in the north of Sinaloa Genny Llaven Valencia1§ Alberto Borbon Gracia2 Xochil Militza Ochoa Espinoza2 Oralia Antuna Grijalva3 Aidé Hernández Hernández3 José Luis Coyac Rodríguez3 1Valle Del Fuerte Experimental Field-INIFAP. Mexico-Nogales International Highway km 1609, Col. Juan José Ríos, Guasave, Sinaloa, Mexico. CP. 81110. 2Experimental Field Norman E. Borlaug-INIFAP. Dr. Norman E. Borlaug Street, km 12, Col. Valle del Yaqui, Cajame, Ciudad Obregón, Sonora. CP. 8500. ([email protected]; [email protected]). 3Universidad Autónoma Agraria Antonio Narro. Periférico Raúl López Sánchez and Santa Fe highway, Torreón, Coahuila, México. CP. 27054. ([email protected]; [email protected]). §Corresponding author: [email protected]. Abstract To determine the productivity of castor oil plant in Sinaloa, the influence of two sowing dates, water availability and two planting densities on the grain yield of four castor oil plant hybrids were evaluated, the trial was established at Valley of Fuerte Experimental Field. During the autumn- winter agricultural cycles with sowing date of December 10, 2015 and spring-summer 2015-2016 with sowing date of February 18, 2016. A randomized complete block design with four replications was used, the plot experimental was four furrows of 20 m long, with a separation of 0.80 m, equivalent to 64 m2, two population densities were managed: 23 000 and 26 000 plants ha-1, and two irrigation regimes per test. The statistical analysis indicated that hybrids 2B-5, Chinatan and HB-8, were higher in yield and without statistical differences, with days at maturity from 145 to 152, so they are considered normal cycle; the average height was 20 m, considered average. -

Human Reproductive Systems Males Vs. Females Learning Goals • Students Will Describe the Basic Anatomy and Physiology of the Male and Female Reproductive Systems
Human Reproductive Systems Males vs. Females Learning Goals • Students will describe the basic anatomy and physiology of the male and female reproductive systems. Gonads are sex organs that create gametes? & excrete sex hormones Gonads are sex organs that create gametes & excrete sex hormones Male gonads are called testes Female gonads are called ovaries -Are the site of sperm production -Are the site of egg production & maturation Gametes are also called sex ?cells, and are used to create offspring with a mixture of genetic information. Gametes are also called sex cells, and are used to create offspring with a mixture of genetic information. Male gametes are called sperm Female gametes are called -produce 300-500 million per 5ml eggs/ova of semen -70,000-100,000 at birth -release 1-2 per month from puberty to menopause. Sex Hormones are chemical? signals that tell the sex organs how to function. Sex Hormones are chemical signals that tell the sex organs how to function. Male hormone is called Female hormones are estrogen testosterone and progesterone -released from the testes -released from the ovary -controls sperm production -controls egg production & release Duct systems help deliver gametes from gonads and are the site of fertilization in females and delivers sperm out of the body in males. Male duct systems include: Epididymis -site of sperm maturation (about 20 days for sperm to mature) Male duct systems include: Vas deferens -Tube for sperm to travel through as they leave the testes Male duct systems include: Urethra -shared tube for release of semen from reproductive tract and urine from the bladder. -

Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho
Algal Sex Determination and the Evolution of Anisogamy James Umen, Susana Coelho To cite this version: James Umen, Susana Coelho. Algal Sex Determination and the Evolution of Anisogamy. Annual Review of Microbiology, Annual Reviews, 2019, 73 (1), 10.1146/annurev-micro-020518-120011. hal- 02187088 HAL Id: hal-02187088 https://hal.sorbonne-universite.fr/hal-02187088 Submitted on 17 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Microbiol. 2019. 73:X–X https://doi.org/10.1146/annurev-micro-020518-120011 Copyright © 2019 by Annual Reviews. All rights reserved Umen • Coelho www.annualreviews.org • Algal Sexes and Mating Systems Algal Sex Determination and the Evolution of Anisogamy James Umen1 and Susana Coelho2 1Donald Danforth Plant Science Center, St. Louis, Missouri 63132, USA; email: [email protected] 2Sorbonne Université, UPMC Université Paris 06, CNRS, Algal Genetics Group, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, CS 90074, F-29688, Roscoff, France [**AU: Please write the entire affiliation in French or write it all in English, rather than a combination of English and French**] ; email: [email protected] Abstract Algae are photosynthetic eukaryotes whose taxonomic breadth covers a range of life histories, degrees of cellular and developmental complexity, and diverse patterns of sexual reproduction. -

Meiosis & Sexual Reproduction Heyer 1
Meiosis & Sexual Reproduction Meiosis & Sex Cells Arise From Preexisting Cells I. Asexual (Mitotic) Reproduction a. Mitosis: production of two identical nuclei b. Cytokinesis: physical division of the cell into two II. Sexual (Meiotic) Reproduction a. Meiosis: production of four non-identical nuclei b. Cytokinesis: physical division of the cell c. Fertilization: fusion of two Sexual reproduction creates sex cells d. Syngamy: fusion of new combinations of alleles. two nuclei Diploid cells have Homologous chromosomes [Homologs]: homologous pairs of chromosomes: same loci, maybe different alleles. 1 set from mom, 1 set from dad. Meiosis: Reductive Division Sex = Meiosis + Syngamy — Reduces Chromosome Number in Half Meiosis has 2 consecutive divisions – Meiosis I: Homologous pairs separate – Meiosis II: Sister chromatids separate Each division has a prophase, metaphase, anaphase and a telophase Meiosis: Syngamy: 2n 1n 1n 2n Diploid haploid Haploid diploid Heyer 1 Meiosis & Sexual Reproduction Chromosomes Matched in Sexual Life Cycles Homologous Pairs (Homologs) Human somatic (body) cells – 23 pairs = 46 chromosomes – Homolog = same size, shape, centromere, and genes Pairs #1 - 22 = Autosomes – Both male and female Pair #23 = Sex Chromosomes – Determine gender – XX = female, XY = male Diploid life history Alternation of Generations Haploid life history (animals) (plants) (fungi) Human karyotype Somatic (body) cells are Diploid Meiosis I Gametes (sex cells) are Haploid Diploid (2n) Prophase I – Two of each kind of chromosome Chromosomes -

Castor Oil Plant (Ricinus Communis)
Invasive plant Castor oil plant Ricinus communis Castor oil plant Ricinus communis Castor oil plant spreads over sandy soil areas, creek banks Also, small amounts of the plant will induce an immunity and gullies. This can lead to a significant loss of prime to poisoning. grazing land. The seeds of castor oil contain ricin, a poison that is Legal requirements extremely toxic to livestock and humans. Leaves have a Castor oil is not a prohibited or restricted invasive lesser amount of toxin. Symptoms of poisoning in animals plant under the Biosecurity Act 2014. However, by law, usually do not appear for a few hours or several days. everyone has a general biosecurity obligation (GBO) to take reasonable and practical steps to minimise the Seeds cause gastrointestinal disorders and leaves tend risks associated with invasive plants under their control. to cause neuromuscular disorders. Poisoning in livestock is rarely reported though, as castor oil plant is seldom grazed by stock when other pasture plants are available. Local governments must have a biosecurity plan that covers lobes; and has small, smooth fruits found in clusters in the invasive plants in their area. This plan may include actions upper parts of the plant. to be taken on certain species. Some of these actions may be required under local laws. Contact your local government Habitat and distribution for more information. Castor oil plant is native to Africa and Asia, and is now Description naturalised throughout Australia. It is often abundant along watercourses and floodplains, disturbed or waste land, and Castor oil plant is a tall, branching perennial shrub that roadsides. -

Emulsions and the HLB System
2393 Blaine Avenue Orono, MN 55391 (952) 906-0771 CONVERGENT COSMETICS FAX: (952) 906-9781 www.ConvergentCosmetics.com Emulsions and the HLB System All creams and lotions have one thing in common. They are both emulsions. An emulsion is a system of two (or more) immiscible materials (usually liquids) in which one material (the dispersed/internal phase) is suspended or dispersed throughout another material (the continuous/external phase) in separate droplets. Most emulsions fall into two different classes, oil in water emulsions and water in oil emulsions. In oil in water emulsions, we have hundreds of tiny oil droplets surrounded by water. In water in oil emulsions, we have the opposite situation. We have hundreds of water droplets surrounded by oil. One of the simplest emulsions is a simple vinegar and oil salad dressing. One of the problems with this simple emulsion is that the oil and vinegar don’t mix. To emulsify the vinegar into the oil, we can use an egg yolk. Egg yolks contain a natural emulsifier call Lecithin. Most creams and lotions on the market today are oil in water emulsions. 2393 Blaine Avenue Orono, MN 55391 (952) 906-0771 CONVERGENT COSMETICS FAX: (952) 906-9781 www.ConvergentCosmetics.com In 1949, William C. (Bill) Griffin developed the Hydrophile-Lipophile Balance System or HLB System when he was a chemist at the Atlas Powder Company, which eventually became ICI Surfactants and is part of Uniqema today. All emulsifier have two parts; like a bar magnet. A bar magnet has a north pole and a south pole. Nonionic emulsifiers also have two poles or parts. -

The Diversity of Plant Sex Chromosomes Highlighted Through Advances in Genome Sequencing
G C A T T A C G G C A T genes Review The Diversity of Plant Sex Chromosomes Highlighted through Advances in Genome Sequencing Sarah Carey 1,2 , Qingyi Yu 3,* and Alex Harkess 1,2,* 1 Department of Crop, Soil, and Environmental Sciences, Auburn University, Auburn, AL 36849, USA; [email protected] 2 HudsonAlpha Institute for Biotechnology, Huntsville, AL 35806, USA 3 Texas A&M AgriLife Research, Texas A&M University System, Dallas, TX 75252, USA * Correspondence: [email protected] (Q.Y.); [email protected] (A.H.) Abstract: For centuries, scientists have been intrigued by the origin of dioecy in plants, characterizing sex-specific development, uncovering cytological differences between the sexes, and developing theoretical models. Through the invention and continued improvements in genomic technologies, we have truly begun to unlock the genetic basis of dioecy in many species. Here we broadly review the advances in research on dioecy and sex chromosomes. We start by first discussing the early works that built the foundation for current studies and the advances in genome sequencing that have facilitated more-recent findings. We next discuss the analyses of sex chromosomes and sex-determination genes uncovered by genome sequencing. We synthesize these results to find some patterns are emerging, such as the role of duplications, the involvement of hormones in sex-determination, and support for the two-locus model for the origin of dioecy. Though across systems, there are also many novel insights into how sex chromosomes evolve, including different sex-determining genes and routes to suppressed recombination. We propose the future of research in plant sex chromosomes should involve interdisciplinary approaches, combining cutting-edge technologies with the classics Citation: Carey, S.; Yu, Q.; to unravel the patterns that can be found across the hundreds of independent origins. -

Section 6: Sex Cells and Fertilisation
S ection 6: S ex Cells and Fertilisation U se the w ords in the w ord bank below to com plete the sentences below : S maller, vagina, anther, halved, fertilisation, nucleus, male, half, gametes, D N A , stigma, female, ovules, pollen, pollen tube, four, zygote, threadlike, one, identical, genes, amino acids, protein, function, meiosis, sex chromosomes, male S ome plants reproduce sexually. T he sexual parts are inside the flow ers. M ost flow ering plants have flow ers w ith both __ ___ __ and _ ___ __ parts. T hese sexual parts produce special sex cells called _ ____ ___ _. Label the diagram above. T he male part of a flow ering plant is called the ___ ___ ___ _ and produces __ ______. T he female part is called the _ ___ ___ _ and produces ovules. Pollen grains are __ ___ ____ and more numerous than ovules, w hich are larger. Fertilisation in flow ering plants occurs by pollen trains being transferred to the _ ___ ___ _. A _____ ___ __ _____then grow s dow n into the ovary and into an ovule. A male gamete then passes dow n the tube and fuses w ith egg cell. T his process is called 1 __ __________. T he fertilised egg is now called a ___ ___ __. Fertilisation produces variety in the offspring because genetically identical gametes form in different w ays, producing different combinations. S exual Reproduction In H umans Label the follow ing diagrams: 2 In humans, fertilisation takes place in the oviduct. -
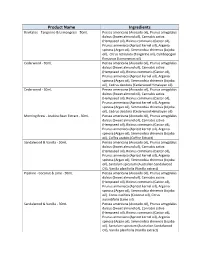
Product Name Ingredients
Product Name Ingredients Revitalise - Tangerine & Lemongrass - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Citrus reticulata (Tangerine oil), Cymbopogon flexuosus (Lemongrass oil) Cedarwood - 30mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Cedrus deodora (Cedarwood Himalayan oil) Cedarwood - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Cedrus deodora (Cedarwood Himalayan oil) Morning Brew - Arabica Bean Extract - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba oil), Coffea arabics (Coffee Extract) Sandalwood & Vanilla - 50mL Persea americana (Avocado oil), Prunus amygdalus dulcus (Sweet almond oil), Cannabis sativa (Hempseed oil), Ricinus communis (Castor oil), Prunus armeniaca (Apricot kernel oil), Argania spinosa (Argan oil), Simmondsia chinensis (Jojoba -

Foxl3, a Sexual Switch in Germ Cells, Initiates Two Independent Molecular Pathways for Commitment to Oogenesis in Medaka
foxl3, a sexual switch in germ cells, initiates two independent molecular pathways for commitment to oogenesis in medaka Mariko Kikuchia, Toshiya Nishimuraa,1, Satoshi Ishishitab, Yoichi Matsudab,c, and Minoru Tanakaa,2 aDivision of Biological Science, Graduate School of Science, Nagoya University, 464-8602 Nagoya, Japan; bAvian Bioscience Research Center, Graduate School of Bioagricultural Sciences, Nagoya University, 464-8601 Nagoya, Japan; and cDepartment of Animal Sciences, Graduate School of Bioagricultural Sciences, Nagoya University, 464-8601 Nagoya, Japan Edited by John J. Eppig, The Jackson Laboratory, Bar Harbor, ME, and approved April 8, 2020 (received for review October 23, 2019) Germ cells have the ability to differentiate into eggs and sperm and elegans (8–10). To date, however, neither protein has been im- must determine their sexual fate. In vertebrates, the mechanism of plicated in germline feminization. commitment to oogenesis following the sexual fate decision in During oogenesis in mice, several transcription factors facili- germ cells remains unknown. Forkhead-box protein L3 (foxl3)isa tate folliculogenesis: lhx8 (LIM homeobox 8) and figla (factor in switch gene involved in the germline sexual fate decision in the the germline alpha) regulate differentiation of primordial follicles teleost fish medaka (Oryzias latipes). Here, we show that foxl3 or- (11–14), and nobox (newborn ovary homeobox), which acts ganizes two independent pathways of oogenesis regulated by REC8 downstream of Lhx8, is indispensable for the formation of sec- meiotic recombination protein a (rec8a), a cohesin component, and ondary follicles (12, 15). It remains unknown how these tran- F-box protein (FBP)47(fbxo47), a subunit of E3 ubiquitin ligase. -

Arizona Administrative Code Between the Dates of October 1, 2020 Through December 31, 2020 (Supp
3 A.A.C. 4 Supp. 20-4 December 31, 2020 Title 3 TITLE 3. AGRICULTURE CHAPTER 4. DEPARTMENT OF AGRICULTURE - PLANT SERVICES DIVISION The table of contents on the first page contains quick links to the referenced page numbers in this Chapter. Refer to the notes at the end of a Section to learn about the history of a rule as it was published in the Arizona Administrative Register. Sections, Parts, Exhibits, Tables or Appendices codified in this supplement. The list provided contains quick links to the updated rules. This Chapter contains rule Sections that were filed to be codified in the Arizona Administrative Code between the dates of October 1, 2020 through December 31, 2020 (Supp. 20-4). Table 1. Fee Schedule ......................................................47 Questions about these rules? Contact: Name: Brian McGrew Address: Department of Agriculture 1688 W. Adams St. Phoenix, AZ 85007 Telephone: (602) 542-3228 Fax: (602) 542-1004 E-mail: [email protected] Website: https://agriculture.az.gov/plantsproduce/industrial- hemp-program The release of this Chapter in Supp. 20-4 replaces Supp. 20-3, 1-50 pages Please note that the Chapter you are about to replace may have rules still in effect after the publication date of this supplement. Therefore, all superseded material should be retained in a separate binder and archived for future reference. i PREFACE Under Arizona law, the Department of State, Office of the Secretary of State (Office), accepts state agency rule filings and is the publisher of Arizona rules. The Office of the Secretary of State does not interpret or enforce rules in the Administrative Code. -

History of Sex Education Table of Contents
History of Sex Education Table of contents Introduction 5 The social hygiene movement 9 Schools and character-building organizations 13 The influence of WWI 17 Moving beyond disease prevention 19 Family life education 21 The sexual revolution and culture wars 25 Controversies erupt 29 The culture wars 33 AIDS changes the debate 37 The fight between abstinence-only and comprehensive sexuality education 39 The rise of the abstinence-only movement 43 Abstinence-only programs face criticism 49 Evidence-based programs and beyond 51 The fight continues 55 Looking forward: Sex ed as a vehicle for social change 59 2 3 Introduction Sex education in the United States has great potential to educate both individuals and society. It can give us knowledge about our bodies; debunk harmful stereotypes about sex, race, and gender; provide opportunities for us to think critically about our own values and relationships; and empower us to stand up for our rights and the rights of others to pleasure, bodily autonomy, and consent. This was not, however, what sex education was initially designed to do. Too often, over the past 100 years of American history, it has been used to do just the opposite. 4 5 Those who originally pushed the importance of SIECUS believes, however, that sex education— educating the public about sexuality did so out if done properly—has the power to serve as a of a fear that their comfortable, white, middle vehicle for social change. Understanding the class way of living was being threatened by the history of sex education in this country, the loosening of sexual morals.