1 BILLING CODE 4410-09-P DEPARTMENT of JUSTICE Drug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5 Part 1300—Definitions
PART 1300—DEFINITIONS (7) 5-androstenediol (3b,17b-dihydroxy- androst-5-ene) Sec. (8) 1-androstenedione ([5a]-androst-1- 1300.01 Definitions relating to controlled en-3,17-dione) substances. (9) 4-androstenedione (androst-4-en- 1300.02 Definitions relating to listed chemi- 3,17-dione) cals. (10) 5-androstenedione (androst-5-en- 1300.03 Definitions relating to electronic or- 3,17-dione) ders for controlled substances and elec- tronic prescriptions for controlled sub- (11) bolasterone (7a,17a-dimethyl-17b- stances. hydroxyandrost-4-en-3-one) 1300.04 Definitions relating to the dis- (12) boldenone (17b-hydroxyandrost-1,4- pensing of controlled substances by diene-3-one) means of the Internet. (13) boldione (androsta-1,4-diene-3,17- 1300.05 Definitions relating to the disposal dione) of controlled substances. (14) calusterone (7b,17a-dimethyl-17b- AUTHORITY: 21 U.S.C. 802, 821, 822, 829, hydroxyandrost-4-en-3-one) 871(b), 951, 958(f). (15) clostebol (4-chloro-17b- SOURCE: 62 FR 13941, Mar. 24, 1997, unless hydroxyandrost-4-en-3-one) otherwise noted. (16) dehydrochloromethyltestosterone (4-chloro-17b-hydroxy-17a-methyl- § 1300.01 Definitions relating to con- androst-1,4-dien-3-one) trolled substances. (17) desoxymethyltestosterone (17a- (a) Any term not defined in this part methyl-5a-androst-2-en-17b-ol) shall have the definition set forth in (a.k.a. ‘madol‘) section 102 of the Act (21 U.S.C. 802), (18) D1-dihydrotestosterone (a.k.a.‘1- except that certain terms used in part testosterone‘) (17b-hydroxy-5a- 1316 of this chapter are defined at the androst-1-en-3-one) beginning of each subpart of that part. -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

A10 Anabolic Steroids Hardcore Info
CONTENTS GENERAL INFORMATION 3 Anabolic steroids – What are they? 4 How do they Work? – Aromatisation 5 More molecules – More problems 6 The side effects of anabolic steroids 7 Women and anabolic steroids 8 Injecting steroids 9 Abscesses – Needle Exchanges 10 Intramuscular injection 11 Injection sites 12 Oral steroids – Cycles – Stacking 13 Diet 14 Where do steroids come from? Spotting a counterfeit 15 Drug Information – Drug dosage STEROIDS 16 Anadrol – Andriol 17 Anavar – Deca-Durabolin 18 Dynabolon – Durabolin – Dianabol 19 Esiclene – Equipoise 20 Primobolan Depot – Proviron – Primobolan orals – Pronobol 21 Sustanon – Stromba, Strombaject – Testosterone Cypionate Testosterone Enanthate 22 Testosterone Propionate – Testosterone Suspension 23 Trenbolone Acetate – Winstrol OTHER DRUGS 24 Aldactone – Arimidex 25 Clenbuterol – Cytomel 26 Ephedrine Hydrochloride – GHB 27 Growth Hormone 28 Insulin 30 Insulin-Like Growth Factor-1 – Human Chorionic Gonadotrophin 31 Tamoxifen – Nubain – Recreational Drugs 32 Steroids and the Law 34 Glossary ANABOLIC STEROIDS People use anabolic steroids for various reasons, some use them to build muscle for their job, others just want to look good and some use them to help them in sport or body building. Whatever the reason, care needs to be taken so that as little harm is done to the body as possible because despite having muscle building effects they also have serious side effects especially when used incorrectly. WHAT ARE THEY? Anabolic steroids are man made versions of the hormone testosterone. Testosterone is the chemical in men responsible for facial hair, deepening of the voice and sex organ development, basically the masculine things Steroids are in a man. used in medicine to treat anaemia, muscle weakness after These masculine effects surgery etc, vascular are called the androgenic disorders and effects of testosterone. -

2010 Prohibited List
The World Anti-Doping Code THE 2010 PROHIBITED LIST INTERNATIONAL STANDARD The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2010 The Prohibited List 2010 19 September 2009 THE 2010 PROHIBITED LIST WORLD ANTI-DOPING CODE Valid 1 January 2010 All Prohibited Substances shall be considered as “Specified Substances” except Substances in classes S1, S2.1 to S2.5, S.4.4 and S6.a, and Prohibited Methods M1, M2 and M3. SUBSTANCES AND METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) PROHIBITED SUBSTANCES S1. ANABOLIC AGENTS Anabolic agents are prohibited. 1. Anabolic Androgenic Steroids (AAS) a. Exogenous* AAS, including: 1-androstendiol (5α-androst-1-ene-3β,17β-diol ); 1-androstendione (5α- androst-1-ene-3,17-dione); bolandiol (19-norandrostenediol); bolasterone; boldenone; boldione (androsta-1,4-diene-3,17-dione); calusterone; clostebol; danazol (17α-ethynyl-17β-hydroxyandrost-4-eno[2,3-d]isoxazole); dehydrochlormethyltestosterone (4-chloro-17β-hydroxy-17α-methylandrosta- 1,4-dien-3-one); desoxymethyltestosterone (17α-methyl-5α-androst-2-en- 17β-ol); drostanolone; ethylestrenol (19-nor-17α-pregn-4-en-17-ol); fluoxymesterone; formebolone; furazabol (17β-hydroxy-17α-methyl-5α- androstano[2,3-c]-furazan); gestrinone; 4-hydroxytestosterone (4,17β- dihydroxyandrost-4-en-3-one); mestanolone; mesterolone; metenolone; methandienone -

Misuse of Drugs (Miscellaneous Amendments) (No. 2) (Jersey) Order 2012 Arrangement
Misuse of Drugs (Miscellaneous Amendments) (No. 2) (Jersey) Order 2012 Arrangement MISUSE OF DRUGS (MISCELLANEOUS AMENDMENTS) (No. 2) (JERSEY) ORDER 2012 Arrangement Article 1 Schedule 2 to the Misuse of Drugs (Jersey) Law 1978 amended .................... 3 2 Schedule to the Misuse of Drugs (Designation) (Jersey) Order 1989 amended .......................................................................................................... 9 3 Misuse of Drugs (General Provisions) (Jersey) Order 2009 amended .......... 11 4 Citation and commencement ......................................................................... 18 ◊ Page - 1 Misuse of Drugs (Miscellaneous Amendments) (No. 2) (Jersey) Order 2012 Article 1 MISUSE OF DRUGS (MISCELLANEOUS AMENDMENTS) (No. 2) (JERSEY) ORDER 2012 Made Coming into force THE MINISTER FOR HEALTH AND SOCIAL SERVICES, in pursuance of Articles 3, 4, 5, 8, 12, 13 and 27 of the Misuse of Drugs (Jersey) Law 1978, and after consultation with the Advisory Council on the Misuse of Drugs, orders as follows – 1 Schedule 2 to the Misuse of Drugs (Jersey) Law 1978 amended (1) In this Article “Law” means the Misuse of Drugs (Jersey) Law 1978. (2) In Part 1 of Schedule 2 to the Law, in paragraph 1(a), after the entry “Sufentanil” there shall be inserted the following entry – “Tapentadol”. (3) In Part 2 of Schedule 2 to the Law – (a) in paragraph 1(a) after the entry “Methylphenobarbitone” there shall be inserted the entry – “Nabilone”; (b) for the full stop at the end of paragraph 1(i) there shall be substituted a semi-colon; and (c) after paragraph 1(i) there shall be added the following sub- paragraphs – “(j) the following substances – 6-(2-aminopropyl)benzofuran 5-(2-aminopropyl)benzofuran; (k) Any compound (not being pipradrol) structurally derived from piperidine, pyrrolidine, azepane, morpholine or pyridine by substitution at a ring carbon atom with a diphenylmethyl group, whether or not the compound is further modified in any of the following ways, that is to say – ◊ Page - 3 Misuse of Drugs (Miscellaneous Amendments) (No. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0144429 A1 Wensley Et Al
US 2014O144429A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0144429 A1 Wensley et al. (43) Pub. Date: May 29, 2014 (54) METHODS AND DEVICES FOR COMPOUND (60) Provisional application No. 61/887,045, filed on Oct. DELIVERY 4, 2013, provisional application No. 61/831,992, filed on Jun. 6, 2013, provisional application No. 61/794, (71) Applicant: E-NICOTINE TECHNOLOGY, INC., 601, filed on Mar. 15, 2013, provisional application Draper, UT (US) No. 61/730,738, filed on Nov. 28, 2012. (72) Inventors: Martin Wensley, Los Gatos, CA (US); Publication Classification Michael Hufford, Chapel Hill, NC (US); Jeffrey Williams, Draper, UT (51) Int. Cl. (US); Peter Lloyd, Walnut Creek, CA A6M II/04 (2006.01) (US) (52) U.S. Cl. CPC ................................... A6M II/04 (2013.O1 (73) Assignee: E-NICOTINE TECHNOLOGY, INC., ( ) Draper, UT (US) USPC ..................................................... 128/200.14 (21) Appl. No.: 14/168,338 (57) ABSTRACT 1-1. Provided herein are methods, devices, systems, and computer (22) Filed: Jan. 30, 2014 readable medium for delivering one or more compounds to a O O Subject. Also described herein are methods, devices, systems, Related U.S. Application Data and computer readable medium for transitioning a Smoker to (63) Continuation of application No. PCT/US 13/72426, an electronic nicotine delivery device and for Smoking or filed on Nov. 27, 2013. nicotine cessation. Patent Application Publication May 29, 2014 Sheet 1 of 26 US 2014/O144429 A1 FIG. 2A 204 -1 2O6 Patent Application Publication May 29, 2014 Sheet 2 of 26 US 2014/O144429 A1 Area liquid is vaporized Electrical Connection Agent O s 2. -

WO 2014/085719 Al 5 June 2014 (05.06.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/085719 Al 5 June 2014 (05.06.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61M 15/00 (2006.01) A24F 47/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 13/072426 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 27 November 2013 (27.1 1.2013) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 61/730,738 28 November 2012 (28. 11.2012) US 61/794,601 15 March 2013 (15.03.2013) US (84) Designated States (unless otherwise indicated, for every 61/83 1,992 6 June 2013 (06.06.2013) us kind of regional protection available): ARIPO (BW, GH, 61/887,045 4 October 201 3 (04. -

2019 Prohibited List
THE WORLD ANTI-DOPING CODE INTERNATIONAL STANDARD PROHIBITED LIST JANUARY 2019 The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2019 SUBSTANCES & METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) IN ACCORDANCE WITH ARTICLE 4.2.2 OF THE WORLD ANTI-DOPING CODE, ALL PROHIBITED SUBSTANCES SHALL BE CONSIDERED AS “SPECIFIED SUBSTANCES” EXCEPT SUBSTANCES IN CLASSES S1, S2, S4.4, S4.5, S6.A, AND PROHIBITED METHODS M1, M2 AND M3. PROHIBITED SUBSTANCES NON-APPROVED SUBSTANCES Mestanolone; S0 Mesterolone; Any pharmacological substance which is not Metandienone (17β-hydroxy-17α-methylandrosta-1,4-dien- addressed by any of the subsequent sections of the 3-one); List and with no current approval by any governmental Metenolone; regulatory health authority for human therapeutic use Methandriol; (e.g. drugs under pre-clinical or clinical development Methasterone (17β-hydroxy-2α,17α-dimethyl-5α- or discontinued, designer drugs, substances approved androstan-3-one); only for veterinary use) is prohibited at all times. Methyldienolone (17β-hydroxy-17α-methylestra-4,9-dien- 3-one); ANABOLIC AGENTS Methyl-1-testosterone (17β-hydroxy-17α-methyl-5α- S1 androst-1-en-3-one); Anabolic agents are prohibited. Methylnortestosterone (17β-hydroxy-17α-methylestr-4-en- 3-one); 1. ANABOLIC ANDROGENIC STEROIDS (AAS) Methyltestosterone; a. Exogenous* -

Metabolic Study of Prostanozol Using Human Liver Microsomes and Humanized Mice
Lecture MANFRED DONIKE WORKSHOP Leen Lootens1, Lore Geldof1, Lieselot Decroix1, Koen Deventer1, Francesco Botrè2, Geert Leroux-Roels3, Philip Meuleman3, Peter Van Eenoo1 Metabolic study of Prostanozol using human liver microsomes and humanized mice DoCoLab, Ghent University, Zwijnaarde, Belgium1; Laboratorio Antidoping, FMSI, Rome, Italy2; CEVAC, Ghent University, Gent, Belgium3 Abstract The first part of the study involved the clean up of the nutritional supplement called Orastan-E from Gaspari Nutrition. Analysis of the supplement Orastan-E content, after the clean up procedure, was performed by GC-MS and it indicated the presence of the anabolic steroid prostanozol. This could be confirmed by comparison with the commercially available pure reference standard of this steroid. In the second part of this study the metabolism of the anabolic steroid prostanozol was investigated using human liver microsomes (in vitro) and a mouse model with a humanized liver (in vivo). Most steroids are subject to metabolic transformations in the human body to enhance their urinary excretion. Prostanozol was administered to uPA+/+-SCID mice which harbour human hepatocytes in their liver and to non-chimeric mice (without transplanted human hepatocytes) as a control. Previously, it was shown that the humanized liver of these mice has functional phase I and II metabolizing enzymes. The extracted mouse urines were analysed with a GC-MS scan method and a LC-MS/MS precursor ion scanning method. The results of pre- and post-administration mouse urines were evaluated. In combination with the results of the microsomal in vitro incubations with the prostanozol reference standard, a complete overview of the prostanozol metabolism was obtained. -

Synthetic Androgens As Designer Supplements
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2015, 13, 89-100 89 Synthetic Androgens as Designer Supplements Jan Felix Joseph and Maria Kristina Parr* Institute of Pharmacy, Freie Universität Berlin, Königin-Luise-Str. 2+4, 14195 Berlin, Germany Abstract: Anabolic androgenic steroids (AAS) are some of the most common performance enhancing drugs (PED) among society. Despite the broad spectrum of adverse effects and legal consequences, AAS are illicitly marketed and distributed in many countries. To circumvent existing laws, the chemical structure of AAS is modified and these designer steroids are sold as nutritional supplements mainly over the Internet. Several side effects are linked with AAS abuse. Only little is known about the pharmacological effects and metabolism of unapproved steroids due to the absence of clinical studies. The large number of designer steroid findings in dietary supplements and the detection of new compounds combined with legal loopholes for their distribution in many countries Maria Kristina Parr show that stricter regulations and better information policy are needed. Keywords: AAS, designer steroids, dietary supplements, performance enhancing drugs. INTRODUCTION drugs and their health consequences earlier this year [10]. This review focuses on the steroidal findings of androgens in The use of anabolic androgenic steroids is a widespread dietary supplements. issue not only among athletes but also in recreational sports as well as in the general population. In elite sports, the use of STEROID HORMONES anabolic agents is prohibited by the World Anti-Doping Agency (WADA). Its list of prohibited substances covers Steroid hormones are lipophilic substances derived from cholesterol and are subgrouped based on their phar- exogenous synthetic steroids as well as endogenous macological profile and their receptor binding affinity as sex androgens and precursors of androgens [1]. -

Pros and Cons Controversy on Molecular Imaging and Dynamic
Open Access Archives of Biotechnology and Biomedicine Research Article Pros and Cons Controversy on Molecular Imaging and Dynamics of Double- ISSN Standard DNA/RNA of Human Preserving 2639-6777 Stem Cells-Binding Nano Molecules with Androgens/Anabolic Steroids (AAS) or Testosterone Derivatives through Tracking of Helium-4 Nucleus (Alpha Particle) Using Synchrotron Radiation Alireza Heidari* Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA *Address for Correspondence: Dr. Alireza Abstract Heidari, Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, In the current study, we have investigated pros and cons controversy on molecular imaging and dynamics USA, Email: of double-standard DNA/RNA of human preserving stem cells-binding Nano molecules with Androgens/ [email protected]; Anabolic Steroids (AAS) or Testosterone derivatives through tracking of Helium-4 nucleus (Alpha particle) using [email protected] synchrotron radiation. In this regard, the enzymatic oxidation of double-standard DNA/RNA of human preserving Submitted: 31 October 2017 stem cells-binding Nano molecules by haem peroxidases (or heme peroxidases) such as Horseradish Peroxidase Approved: 13 November 2017 (HPR), Chloroperoxidase (CPO), Lactoperoxidase (LPO) and Lignin Peroxidase (LiP) is an important process from Published: 15 November 2017 both the synthetic and mechanistic point of view. Copyright: 2017 Heidari A. This is an open access article distributed under the Creative -
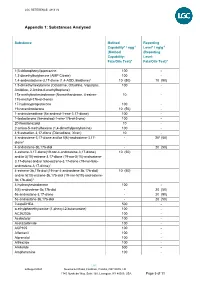
Appendix 1: Substances Analysed
LGC REFERENCE: 4813 V2 Appendix 1: Substances Analysed Substance Method Reporting Capability* / ngg-1 Level* / ng/g-1 (Method (Reporting Capability: Level: Fats/Oils Test)4 Fats/Oils Test)4 1(3-chlorophenyl)piperazine 100 - 1,3-dimethylbutylamine (AMP Citrate) 100 - 1,4-androstadiene-3,17-dione (1,4-ADD, Boldione)2 10 (50) 10 (50) 1,5-dimethylhexylamine (Octodrine, Ottodrina, Vaporpac, 100 - Amidrine, 2-Amino-6-methylheptane) 17a-methylnortestosterone (Normethandrone, 4-estren- 10 - 17a-methyl-17b-ol-3-one) 17-hydroxyprogesterone 100 - 19-norandrosterone 10 (50) - 1-androstenedione (5a-androst-1-ene-3,17-dione) 100 - 1-testosterone (5a-androst-1-ene-17b-ol-3-one) 100 - 20-Norstanozolol 10 - 2-amino-5-methylhexane (1,4-dimethylpentylamine) 100 - 4,9-estradien-3,17-dione (Dienedione, Xtren) 10 - 4-androstene-3,17-dione and/or 5(6)-androstene-3,17- - 203 (50) dione1 4-androstene-3b,17b-diol - 20 (50) 4-estrene-3,17-dione(19-nor-4-androstene-3,17-dione) 10 (50) and/or 5(10)-estrene-3,17-dione (19-nor-5(10)-androstene- 3,17-dione) and/or 5(6)-estrene-3,17-dione (19-nor-5(6)- androstene-3,17-dione)1 4-estrene-3b,17b-diol (19-nor-4-androstene-3b,17b-diol) 10 (50) and/or 5(10)-estrene-3b,17b-diol (19-nor-5(10)-androstene- 3b,17b-diol)1 4-hydroxytestosterone 100 - 5(6)-androstene-3b,17b-diol - 20 (50) 5a-androstane-3,17-dione - 20 (50) 5a-androstane-3b,17b-diol - 20 (50) 7-ketoDHEA 500 - α-ethylphenethylamine (1-phenyl-2-butanamine) 100 - AC262536 100 - Acebutolol 100 - Acetazolamide 100 - ACP105 100 - Alfentanil 100 - Alprenolol 100 - Althiazide