Nail Abnormalities Identified in an Aging Study of 30 Inbred Mouse Strains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Another Rashmanaging ? Common Skin Problems in Primary Care: Ugh….Another Rash Kathleen Haycraft, DNP, FNP/PNP-BC, DCNP, FAANP Objectives
Another RashManaging ? Common Skin Problems in Primary Care: Ugh….Another Rash Kathleen Haycraft, DNP, FNP/PNP-BC, DCNP, FAANP Objectives At the completion of this session the learner will be able to: 1. Identify common skin rashes seen in dermatology 2. Differentiate between rashes that require urgent treatment and those that require monitored therapy. 3. Determine an appropriate treatment plan for common rashes Financial Disclosures and COI The speaker is on the advisory committee for: ABVIE CELGENE LILLY NOVARTIS PFIZER VALEANT Significance Dermatologic conditions are the number one reason to enter ambulatory walk in clinics The skin it the largest organ of the body and frequently is a measure of what is occurring internally Take a good history Duration What did it look like in the beginning and how has it progressed? Does anyone else in your immediate family or workers have a similar rash? Have you been ill and in what way? What have you treated the rash with prescription or over the counter medications? Take a good history Have they seen anyone and what diagnosis where you given? What is your medical history? What medicines do you take? Does it itch, hurt, scale, or asymptomatic? Give it a scale. How did it begin and what does has it changed (tie this into treatment history)? Is the patient sick? What does it looks like? Macule vs. Patch Papule, nodule, pustule, tumor Vesicle or Bulla Petechial or purpura Indurated vs. non-indurated Is it crusted…deep or superficial What pattern…. Blaschkos vs. dermatome,, symmetrical, central vs. caudal, reticular, annular vs. -
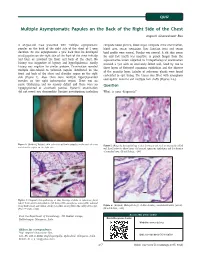
Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao
QUIZ Multiple Asymptomatic Papules on the Back of the Right Side of the Chest Angoori Gnaneshwar Rao A 43-year-old male presented with multiple asymptomatic complete blood picture, blood sugar, complete urine examination, papules on the back of the right side of the chest of 1 year blood urea, serum creatinine, liver function tests and serum duration. He was asymptomatic a year back then he developed lipid profile were normal. Fundus was normal. A slit skin smear small papules on the right side of the front of the chest initially for acid fast bacilli was negative. A punch biopsy from the and later on involved the front and back of the chest. No representative lesion subjected to histopathological examination history was suggestive of leprosy and hyperlipidemias. Family revealed a cyst with an intricately folded wall, lined by two to history was negative for similar problem. Examination revealed three layers of flattened squamous epithelium and the absence multiple skin-colored to yellowish papules distributed on the of the granular layer. Lobules of sebaceous glands were found front and back of the chest and shoulder region on the right embedded in cyst lining. The lumen was filled with amorphous side [Figure 1]. Also, there were multiple hyperpigmented eosinophilic material and multiple hair shafts [Figures 2-4]. macules on the right infrascapular region. There was no nerve thickening and no sensory deficit and there were no Question hypopigmented or anesthetic patches. Systemic examination did not reveal any abnormality. Routine investigations including What is your diagnosis? (Original) Multiple skin-colored to yellowish papules on the back of chest Figure 1: Figure 2: (Original) Histopathology of skin showing a cyst with an intricately folded and shoulder region on the right side wall lined by two to three layers of flattened squamous epithelium and the absence of granular layer. -

Metastasis of Meningioma: a Rare Differential Diagnosis In
logy: Op go en n y A Lunger et al., Otolaryngol (Sunnyvale) 2017, 7:6 r c a c l e o s t DOI: 10.4172/2161-119X.1000333 s O Otolaryngology: Open Access ISSN: 2161-119X Case Report OpenOpen Access Access Metastasis of Meningioma: A Rare Differential Diagnosis in Subcutaneous Masses of the Scalp Alexander Lunger1*, Tarek Ismail1#, Adrian Dalbert2, Kirsten Mertz3, Thomas Weikert4, Dirk Johannes Schaefer1 and Ilario Fulco1 1Department of Plastic, Reconstructive, Aesthetic and Hand Surgery, University Hospital Basel, Basel, Switzerland 2Department of Otorhinolaryngology-Head and Neck Surgery, University Hospital Zurich, Zurich, Switzerland 3Department of Pathology, Kantonsspital Basel Land, Liestal, Switzerland 4Department of Radiology, University Hospital Basel, Switzerland Abstract Background: Subcutaneous masses of the scalp have a wide range of differential diagnosis. After removal of a meningioma in the patient’s history, scalp metastasis from the previously resected meningioma should be considered. Methods: A 86 year old patient presented with a local swelling on the left temporal forehead and no other clinical symptoms. Eleven years earlier an extra-axial meningioma was resected. The patient was receiving immunosuppressive therapy subsequent to kidney transplantation. After clinical examination and MRI, a lipoma was suspected. The mass was resected under local anesthesia. Results: Histopathology revealed a metastasis of the previously removed meningioma (WHO grade II). No further treatment was recommended. Clinical follow-up was without pathological findings so far. Conclusion: Scalp metastases of meningiomas are a rare finding. However, if patient history reveals removal of a meningioma, scalp metastasis must be a differential diagnosis for subcutaneous masses even years after the initial surgery. -

The Way We Were the New Style Always Get an Informed Consent Always Ask About Allergies!
6/3/2019 The Way We Were The Expanding Optometric Scope: 1045-02-.12 PRIMARY EYE CARE PROCEDURES. For the Minor Surgical Procedures purpose of 1993 Public Acts Chapter 295 • The performance of primary eye care procedures rational to the Needles, Blades and Radio ‐ Waves treatment of conditions or diseases of the eye or eyelid is determined by the board to be those procedures that could be performed in the optometrist’s office or other health care facilities that would require no more than a topical anesthetic. Laser Jason Duncan, OD, FAAO surgery and radial keratotomy are excluded. • Authority: T.C.A. §§4-5-202, 4-5-204, 63-8-12, and Public Diplomate, American Board of Optometry Chapter 295, Acts of 1993. Administrative Associate Professor, Southern College of Optometry • History: Original rule filed February 14, 1993; effective April 30, 1994. The New Style • An optometrist who uses a local anesthetic in the manner allowed by this subsection shall provide to • The use of a local anesthetic in conjunction with the primary care the board of optometry proof that the optometrist treatment of an eyelid lesion; provided, however, no optometrist has current CPR certification by an organization shall use a local anesthetic for this purpose unless that optometrist has met the certification requirements set forth in 63‐8‐112(4) and approved by the board; provide, that the optometrist in the rules of the board of optometry for the administration of may meet this requirement by providing proof to the pharmaceutical agents in the performance of primary eye care procedures. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Epidermoid Cyst: Highlights on Diagnosis and Magnetic Resonance Imaging Features
EpidermoidCase cyst Report Epidermoid cyst: highlights on diagnosis and magnetic resonance imaging features Cisto epidermóide: processo de diagnóstico e características das imagens por ressonância magnética Abstract Larissa Santana Arantes Elias a Angélica Ferreira Oton-Leite a b Purpose: Epidermoid and dermoid cysts are extremely rare developmental cysts of a benign Clóvis Martins Silva a nature, which may occur anywhere in the body, although about 7% are found in the head Rejane Faria Ribeiro-Rotta Aline Carvalho Batista a and neck. This article reports a clinical case of a patient who had an epidermoid cyst and its Elismauro Francisco de Mendonça a magnetic resonance imaging (MRI) features. Case report: This case discusses an epidermoid cyst in a 36-year-old woman complaining about speech difficulty. Clinical examination revealed an extensive swelling on the floor of the mouth. MRI findings showed a cystic homogenous lesion located underneath the mylohyoid a Department of Oral Medicine (Oral Pathology), muscle which was removed by surgery. Histological examination of the mass confirmed the Dental School, Federal University of Goiás, diagnosis of an epidermoid cyst. Goiânia, GO, Brazil b Araújo Jorge Hospital, Association of Cancer Conclusion: We concluded that MRI was considered useful for a more accurate diagnosis Combat of Goiás, Goiânia, GO, Brazil prior to treatment. Key words: Epidermoid cyst; dermoid cyst; magnetic resonance images; differential diagnosis Resumo Proposta: Cistos epidermóide e dermóide são cistos de desenvolvimento extremamente raros, de natureza benigna, que podem ocorrer em qualquer região do corpo e somente 7% são encontrados na região de cabeça e pescoço. Este artigo apresenta o caso clínico de uma paciente que possuía um cisto epidermóide juntamente com as características das imagens por ressonância magnética. -

Solitary Nodule with White Hairs
PHOTO CHALLENGE Solitary Nodule With White Hairs Megan Wetzel, MD, MPH; Amy Gagnon, MD; Joseph McDermott, MD A 72-year-old man presented with a new asymp- tomatic 0.7-cm flesh-colored papule with a cen- tral tuft of white hairs on the posterior scalp. The remainder of the physical examination was unre- markable. Biopsy for histopathologic examination was performed to confirm diagnosis. WHAT’S THEcopy DIAGNOSIS? a. dilated pore of Winer b. epidermoid cyst c. pilar sheath acanthoma d. trichoepitheliomanot e. trichofolliculoma DoPLEASE TURN TO PAGE E2 FOR THE DIAGNOSIS CUTIS Dr. Wetzel was from the University of Vermont, Burlington, and currently is from the Division of Dermatology, Department of Internal Medicine, University of Louisville School of Medicine, Kentucky. Drs. Gagnon and McDermott were from the University of Virginia, Charlottesville. Dr. Gagnon currently is from Dermatology PLC, Charlottesville and Orange, Virginia. Dr. McDermott currently is from the Department of Pathology and Laboratory Services, David Grant Medical Center, Fairfield, California. The authors report no conflict of interest. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Air Force or the Department of Defense. Correspondence: Megan Wetzel, MD, MPH, 3810 Springhurst Blvd, Louisville, KY 40241 ([email protected]). WWW.CUTIS.COM VOL. 100 NO. 2 I AUGUST 2017 E1 Copyright Cutis 2017. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Trichofolliculoma icroscopic examination revealed a dilated cystic Clinically, the differential diagnosis of a flesh-colored follicle that communicated with the skin surface papule on the scalp with prominent follicle includes M(Figure). -

Cysts a Cyst Is a Benign, Round, Dome-Shaped Encapsulated Lesion
Cysts A cyst is a benign, round, dome-shaped encapsulated lesion that contains fluid or semi-fluid material. It may be firm or fluctuant and often distends the overlying skin. There are several types of cyst. The most common are described here. Pseudocyst? Cysts that are not surrounded by a capsule are better known as pseudocysts. These commonly arise in acne. Cysts Epidermoid cysts are due to the proliferation of epidermal cells within the dermis. So basically the layers of skin that are supposed to be on the outside instead invaginate in. Skin is tucked in to form a sack that is lined by healthy epidermal cells that continue to multiply, mature and form keratin. These can be found just about anywhere on the body. A central pore or punctum may be present. Keratinous contents are soft, cheese-like and malodorous. The origin of a trichilemmal cyst is hair root sheath and, thus, these are often found on the scalp and are very firm. Inheritance is autosomal dominant. The origin of steatocystoma is the sebaceous duct within the hair follicle. A solitary steatocystoma is known as steatocystoma simplex. More often, there are multiple lesions (steatocystoma multiplex) on the chest, upper arms, axillae, neck and scrotum or vulva and this is usually an autosomal dominantly inherited disorder. The cysts arise in the late teens and 20s due to the effect of androgens and persist lifelong. They are freely moveable, smooth flesh to yellow color papules 3–30 mm in diameter. There is no central punctum. The content of the cysts is predominantly sebum. -

Objectives Common Cutaneous Bacterial Infections Folliculitis Eosinophilic Folliculitis
Objectives • 1. To implement a management strategy for MRSA Common Cutaneous eradication. • 2. To recognize the common presentations of cutaneous Bacterial Infections syphilis. • 3. To identify cutaneous manifestations of cocksackie virus infection. • 4. To manage tinea versicolor effectively. Eosinophilic Folliculitis Folliculitis (HIV) • Inflammation of hair follicle(s) • Symptoms: Often pruritic (itchy) Pseudomonas folliculitis Treatment of Folliculitis Folliculitis: Causes • Bacterial – culture pustule • Bacteria: – topical clindamycin or oral cephalexin / doxycycline – Gram positives (Staph): most common – shower and change shirt after exercise – Gram negatives: Pseudomonas – “hot tub” folliculitis – keep skin dry; loose clothing • Fungal: Pityrosporum aka Malassezia • Fungal: topical antifungals (e.g., ketoconazole) • HIV: eosinophilic folliculitis (not bacterial) • Eosinophilic folliculitis • Renal Failure: perforating folliculitis (not – Phototherapy bacterial) – Treat the HIV Infectious or Inflammatory? Infectious! 21 year old female with controlled Furunculosis Crohn’s disease and history of • Incise and Drain hidradenitis suppuritiva presents stating • Swab culture • Assume MRSA she has recurrent flares of her HS • Empiric oral antibiotics – Bactrim DS 1 po bid x 10 days – Doxycycline 100mg po bid x 10 days – Augmentin / Cephalexin / Clindamycin if want to roll the dice with close follow-up • Mupirocin ointment – To wounds tid – To nares (bid x 5days) • Bleach bath – 1/3 cup bleach to tub biw-tiw • Wash fomites – bedding, worn -

Congenital Giant Keratinous Cyst Mimicking Lipoma: Case Report and Review Samrat Sabhlok, Ketki Kalele1, Asmita Phirange, Supriya Kheur1
E-IJD CASE REPORT Congenital Giant Keratinous Cyst Mimicking Lipoma: Case Report and Review Samrat Sabhlok, Ketki Kalele1, Asmita Phirange, Supriya Kheur1 Abstract From the Departments of Oral Epidermal cysts represent the most common cutaneous cysts. They arise following a localized and Maxillofacial Surgery, 1 inflammation of the hair follicle and occasionally after the implantation of the epithelium, Oral Pathology and Microbiology, following a trauma or surgery. Conventional epidermal cysts are about 5 cm in diameter; Dr. D.Y. Patil Dental College and Hospital, DPU, Pimpri, Pune, however, rare reports of cysts more than 5 cm are reported in the literature and are referred as Maharashtra, India “Giant epidermal cysts.” Epidermal cysts although common, can mimic other common benign lesions in the head and neck area. A thorough clinico‑pathologic investigation is needed to diagnose these cutaneous lesions as they differ in their biologic behavior, treatment, and Address for correspondence: prognosis. We report a case of a giant epidermoid cyst in the scalp area of a young female Dr. Samrat Sabhlok, patient which mimicked lipoma on clinical, as well as cyotological examination. We also Department of Oral and Maxillofacial Surgery, Dr. D.Y. Patil present a brief review of epidermal cysts, their histopathological differential diagnosis, and Dental College and Hospital, their malignant transformation. DPU, Pimpri, Pune ‑ 411 018, Maharashtra, India. Key Words: Giant epidermal cyst, histopathology, keratinous cyst, scalp E‑mail: [email protected] What was known? Epidermal cysts are the common keratinous cysts of skin. Introduction These epithelial, walled cysts vary from a few millimeters Epidermal cysts represent the most common cutaneous to 5 cm in diameter. -

Epidermoid Cyst
Epidermoid cyst Most commonly known as a sebaceous cyst but also known as epidermoid inclusion cyst, Infundibular cyst, epidermal cyst, epidermal inclusion cyst. What is an epidermoid cyst? An epidermoid cyst is a benign walled-off cavity filled with keratin which originates from the hair follicle unit. What causes an epidermoid cyst? Epidermoid cysts are the most common type of cyst. They may be primary or they may arise from disrupted follicular structures due to trauma or comedone formation (blackheads). Multiple cysts may occur in the conjunction with acne vulgaris, Gardner syndrome and in nevoid basal cell carcinoma syndrome. Tiny superficial epidermoid cysts are known as milia. What does it look like? Epidermoid cysts appear as flesh coloured to yellowish, firm, round nodules of variable size. A central pore or punctum may be present. They are usually syptoless ut soeties disharge a foul sellig, heese-like aterial. Less frequently, the cysts can be painful due to inflammation or infection. How is an epidermoid cyst diagnosed? The diagnosis is usually made by a clinical examination. Sometimes a biopsy may be needed. How is epidermoid cyst treated? . Epidermoid cysts that do not concern a person need not be treated. Inflamed epidermoid cysts may require treatments with antibiotics. Incision, drainage and/or steroid injections may be helpful in rare cases to speed up the resolution of the inflammation. Non-inflamed cysts can be removed surgically and the contents and wall of the cyst drained. However, the cyst may recur if the entire cyst wall is not removed. . -

Common Skin Growths and Tumours
Skin Tags / Fibroepithelial Polyps • Seborrheic keratoses can increase in numbers with Sebaceous Hyperplasia age and with prolonged sun exposure. • Skin tags are common, benign flesh-coloured to • Sebaceous hyperplasia are benign skin growths brown skin growths. • Treatment is usually not necessary unless they made up of enlarged oil (sebaceous) glands. They become itchy, irritated or of cosmetic concern. occur in adults and are more common in males. • There is an increased risk in overweight individuals, Treatment options include cryotherapy (liquid pregnant women and those with other family • They present as small, flesh-coloured to yellow nitrogen), scraping of the skin (curettage or members who have skin tags. bumps, most commonly found on the face. cautery) or laser therapy. • They are commonly found in skin folds such as the • Sometimes, they can mimic skin cancer and a • Sometimes a darkly pigmented seborrheic neck, armpits, and groin, although they can occur small skin biopsy may be needed for microscopic keratosis may look like a cancerous mole. In these Common Skin just about anywhere on the body. examination to confirm the diagnosis. cases, a small skin biopsy may need to be taken Growths and Tumours • Occasionally, they may twist and strangulate their for microscopic examination. The risk of recurrence • Treatment is purely for cosmetic reason, and blood supply, resulting in pain. Trauma and irritation is high. include cryotherapy (liquid nitrogen), electrosurgery can occur from rubbing against clothing or jewelry. or laser ablation. • Skin tags may be left alone. However,they can be Cherry Angioma removed with simple snip/ shave excision, lasers or • Cherry angiomas are small, benign growths made electrocautery.