Precision Diagnostics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curriculum Vitae Professor Dr. Herta Flor
Curriculum Vitae Professor Dr. Herta Flor Name: Herta Flor Main areas of research: pain and phantom phenomena, role of learning/memory processes Herta Flor is distinguished for seminal discoveries in the field of pain and phantom phenomena including the cortical processing of pain-related information in humans. Her research focuses on the interaction of brain and behavior, in particular the question how behavior and experience influence neural processes and how neural processes alter behavior and experience. Academic and Professional Career Since 2000 Scientific Director, Department of Cognitive and Clinical Neuroscience, Central Institute of Mental Health, Mannheim and Professor and Chair for Neuropsychology and Clinical Psychology, Medical Faculty Mannheim, Heidelberg University, Germany 1999 - 2000 Interim Professor (C4) for Clinical Psychology, University of Marburg, Germany 1995 - 2000 Professor and Chair of Clinical Psychology, Department of Psychology, Humboldt- University Berlin, Germany 1993 - 1995 Professor of Clinical and Somatic Psychology, Department of Psychology, Humboldt- University Berlin, Germany 1991 - 1993 Heisenberg Fellow of the Deutsche Forschungsgemeinschaft, Department of Clinical and Physiological Psychology, University of Tübingen, Germany 1990 - 1991 Interim Professor of Clinical Psychology, Department of Psychology, University of Marburg, Germany Nationale Akademie der Wissenschaften Leopoldina www.leopoldina.org 1 1990 Habilitation, Clinical Psychology and Psychophysiology, Faculty of Social and Behavioral -

HAUS Scholarship for Bachelor's Or Master's Students Enrolled at US
Heidelberg University: HAUS Scholarship for Bachelor’s or Master’s Students enrolled at U.S. Universities Heidelberg Alumni U.S. (HAUS) is pleased to invite applications for the HAUS Scholarship. The stipend is available to one qualified student from a United States university planning to study at Universität Heidelberg. The scholarship award is $5,000 per semester for Master’s program students and $3,000 for Bachelor’s program students for the winter semester 2016/2017. We are seeking candidates with a strong academic background who can also fulfill the role of ambassador for HAUS upon their return from their study abroad experience. Universität Heidelberg is consistently ranked as one of the best universities in Germany and one of the top rated institutions of higher education and research in the world. It has been distinguished twice as an elite university in the framework of the German Excellence Initiative. Universität Heidelberg is a research university with a wide range of study programs and a strong international orientation: about one-fifth of the university’s 31,000 students and one-third of its doctoral candidates are foreign nationals. With its landmark castle, Old Town and the Neckar River, Heidelberg ranks among the most beautiful cities in Germany. It is conveniently located just one hour south of Frankfurt International Airport. Heidelberg University Association, 871 United Nations Plaza, New York, NY 10017 Phone: 212-758-3324, [email protected], www.HeidelbergUniversity.org “Having the opportunity to study at one of the most prestigious universities in Germany is both an honor and a privilege. The quality of education is simply outstanding. -

European Public Choice Society 2017
European Public Choice Society 2017 Annual Meeting April 19–22, 2017 School of Public Policy Central European University Budapest, Hungary © 2017, School of Public Policy at CEU Layout: Judit Kovács l Createch Printing: Createch Table of Contents Welcome to Budapest ...................... 3 Scientific Program Snapshot ....... 4 Keynote Addresses .............................. 9 Important Information........................ 12 Venue Locations ..................................... 13 Wicksell Prize ............................................. 16 Format of Parallel Sessions ........... 18 Detailed Scientific Program .......... 19 Social Program ......................................... 40 About Budapest and CEU ............. 43 Former EPCS Presidents ................. 45 EPCS 2017 Organizers ....................... 46 Conference Participants .................. 47 We recycle Please return your magnetic visitor cards when you leave. Please also use the recycling bins that are located on each floor. Thank you. 1 Photo: SPP/Daniel Vegel Welcome to Budapest! We are delighted to welcome all of you to Budapest and to the School of Public Policy at Central European University for the 2017 Annual Meeting of the European Public Choice Society. We are especially pleased to be hosting this important gathering at the University’s new state-of-the-art buildings in downtown Budapest that were inaugurated only last fall. These environmentally sustainable spaces combine the latest technology with attractive design features – including a rooftop garden on -

Baden-Württemberg Exchange Program
Baden-Württemberg Exchange Program Program Overview This program is a North Carolina Exchange program hosted by UNC Greensboro. In this unique program, North Carolina students have the chance to study at one of the Baden-Wuerttemberg Universities in Germany, and in exchange, Baden-Wuerttemberg students have the opportunity to study at one of the participating North Carolina public institutions. Program Facts Application & Eligibility Locations Program Dates *University of Mannheim (Mannheim) (Karlsruhe, Konstanz, Tübingen, and Hohenheim ) Heidelberg University (Heidelberg) Full Academic Year .................... Aug, Sept, or Oct to July *University of Hohenheim (Stuttgart) Spring .........................................Jan, Feb, or April to July *Karlsruhe Institute of Technology (KIT) (Karlsruhe) *University of Konstanz (Konstanz) Application Deadlines University of Stuttgart (Stuttgart) Fall/Academic Year ...................................... Mid-February *University of Tübingen (Tübingen) Spring ......................................................... Early October University of Ulm (Ulm) University of Freiburg *spring options Eligibility • (All but Mannheim) Minimum equivalency of two years of German Type of Program ............................................... Exchange • (Mannheim) Two years of German if taking German Program Dates classes • Must a degree-seeking student (Most Locations) • Have at least sophomore standing Full Academic Year ........................ October to September • Have at least a 2.75 cumulative GPA Spring -
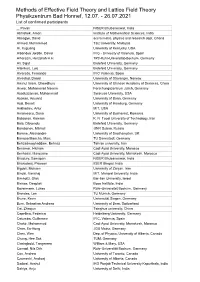
Methods of Effective Field Theory and Lattice Field Theory Physikzentrum Bad Honnef, 12.07
Methods of Effective Field Theory and Lattice Field Theory Physikzentrum Bad Honnef, 12.07. - 26.07.2021 List of confirmed participants _, Pavan NISER Bhubaneswar, India Abhishek, Aman Institute of Mathematical Sciences, India Aboagye, David accra metro, physics and research dept, Ghana Ahmed, Mohammed Taiz University, Malaysia Ai, Xuguang University of Kentucky, USA Albandea Jordán, David IFIC - Univesity of Valencia, Spain Alharazin, Herzallah H.H. TP2-Ruhr-Universität-Bochum, Germany Ali, Sajid Bielefeld University, Germany Altenkort, Luis Bielefeld University, Germany Alvarado, Fernando IFIC Valencia, Spain Alvestad, Daniel University of Stavanger, Norway Aminul Islam, Chowdhury University of Chinese Academy of Sciences, China Anwar, Muhammad Naeem Forschungszentrum Jülich, Germany Asaduzzaman, Muhammad Syracuse University, USA Asokan, Anuvind University of Bonn, Germany Assi, Benoit University of Hamburg, Germany Avkhadiev, Artur MIT, USA Avramescu, Dana University of Bucharest, Romania Babaeian, Kamran K. N. Toosi University of Technology, Iran Bala, Dibyendu Bielefeld University, Germany Barabanov, Mikhail JINR Dubna, Russia Barone, Alessandro University of Southampton, UK Barroso Mancha, Marc TU Darmstadt, Germany Behzadmoghaddam, Behnaz Tehran university, Iran Benamer, Hicham Cadi Ayad University, Morocco Benhassi, Marouane Cadi Ayad University, Marrakesh, Morocco Bhadury, Samapan NISER Bhubaneswar, India Bharadwaj, Praveen IISER Bhopal, India Bigdeli, Mohsen University of Zanjan, Iran Bindal, Vanshaj MIT, Manipal University, India Birnholtz, Ofek Bar-Ilan University, Israel Biswas, Deeptak Bose Institute, India Bovermann, Lukas Ruhr-Universität Bochum, Germany Brandes, Len TU Munich, Germany Brune, Kevin Universität Siegen, Germany Burri, Sebastian Andreas University of Bern, Switzerland Cai, Zhaojun Tsinghua university, China Capellino, Federica Heidelberg University, Germany Catumba, Guilherme IFIC, Valencia, Spain Chakir, Mohammed Cadi Ayad University, Marrakesh, Morocco Chao, En-Hung JGU Mainz, Germany Chen, Wen Dep. -

International Conference for Phd-Students in Diaconic Science
International Conference for PhD-Students in Diaconic Science Dear PhD students, two years ago, we had a first international meeting for PhD students in diaconic science at the Institute for Diaconic Science and Management in Bielefeld, Germany. We would like to invite you today to present papers at the next meeting which will take place from July 19 to July 20, 2019 at the Institute for the Study of Diaconia (Diakoniewissenschaftliches Institut/DWI) at Heidelberg University. In different European countries universities offer PhD-programs with a focus on diaconic science. The objective of the conference is to connect students who are working and studying in these programs to get to know each other, to develop avenues of cooperation and to discuss methodological questions as well as approaches to the “philosophy “ of diaconic science. Thus, we would like to invite you to send in a one page description of your PhD project which you would like to present at the conference (poster or paper presentation, forum of questions and discussion). Please mail the one page description of your project until December 30, 2018 to [email protected] heidelberg.de. Diaconic Science is a young science. It is coming from a theological background and developing an interdisciplinary approach to cover the wide range of topics that are raised by social work and social services by church based organizations or local churches. In many countries these organizations operate in a pluralistic society under the conditions of social markets. The shrinking of churches and the growth of these markets has led to the following question: what is the role theology in the study of diaconia? There will be an opening session focusing on this question. -

German Study Abroad
German Study Abroad German program director in order to learn more about scholarship opportunities to sup5 Why Study port study abroad and about other opportuni5 German Abroad? ties during your studies at 6NCG and a.ter graduation! )Studying abroad is an experience Other benefits... that I will always treasure. I not Simply studying abroad in any country waives a Global mar8er2 and depending on the pro5 only grew as a student but also gram2 a Global Non5Western mar8er. Spea8 to as a whole. ,he adventure o. an IPC adviser .or more in.ormation. living and studying in a di/erent country is unbelievably Sarah Wendland in Germany Will I Graduate on Time? rewarding and challenging at YES! Students receive 6NCG credit .or classes ta8en abroad2 so there is no need to prolong the same time. 0earning the German Major Specifics: graduation. German culture and being When is the best time to study abroad? immersed in the language gave 1ost students aim to study abroad their 3unior year2 Can I Afford It? me a be&er understanding o. .all semester. However not everyone has to do so. YES! On semester or year5long exchange pro5 grams2 students pay regular 6NCG tuition and what opportunities my degree in Any time a.ter the @rst semester at 6NCG students are eligible to apply. It is best to tal8 to an academic .ees. Housing and meal costs are typically German could possibly provide. advisor as well as advisors at the IPC office. Given equivalent to a semester in residence at 6NCG. 1y advice to students the di/erences in academic calendars2 it is best to Any @nancial aid received at 6NCG can be considering studying abroad is study in Germany or Austria either .or the whole year applied to the program costs. -

Information for International Student Applicants
INFORMATION FOR INTERNATIONAL STUDENT APPLICANTS CONTENT Universität Heidelberg 5 Degree programmes and range of subjects 5 Study requirements 10 Proficiency in German 10 Recognition of foreign school-leaving certificates 11 Subject-related requirements 12 Requirements for master’s programmes 12 PhD programmes 13 Recognition of previous study periods 14 Student residence permit 15 Admission restrictions and selection procedures 16 Subjects with admission restrictions 16 Subjects with entrance examination procedure 17 Subjects with access restriction 17 PhD candidates 17 Study preparation at Universität Heidelberg 17 Language preparation 18 Subject preparation 19 Application for admission 21 Undergraduate programmes – first subject-related semester 21 Undergraduate programmes – higher subject-related semester 28 Graduate programmes – first subject-related semester 29 Graduate programmes – higher subject-related semester 30 Short-term studies 31 PhD candidates 32 General application documents 32 Additional documents for undergraduate programmes 33 Additional documents for graduate programmes 34 Additional documents depending on the applicant’s nationality 35 Additional documents for short-term studies 35 Additional documents for transfer students 35 Important notes 36 4/5 Information for international student applicants Universität Heidelberg Degree programmes and range of subjects Financial and social aspects 39 Orientation 39 Semester programme 40 Financial aspects 40 Insurance 41 Accommodation 42 Public transportation 43 Studierendenwerk -

2020 JMK Schools
Jane M. Klausman Women in Business Scholarship List contains the names of Schools/Institutes by Country where recipients have attended (1999-2020) Australia Central Queensland University University of Queensland Edith Cowan University University of South Australia Flinders University of South Australia University of Southern Queensland Griffith University, Australia University of Sydney La Trobe University Bendigo University of Tasmania Monash University University of Technology Sydney Murdoch University University of the Sunshire Queensland University of Technology University of Western Australia University of New England Victoria University University of Newcastle Austria FH-Joanneum University of Applied Sciences Leopold Franzens University Bangladesh Institute of Business Administration University of Dhaka Belgium ICHEC Brussels Management School University of Antwerp Ghent University Bulgaria American University in Bulgaria Canada Acadia University University of Guelph Brock University University of Ottawa Carleton University University of Toronto Concordia University University of Victoria Dalhousie University University of Waterloo McGill University University of Western Ontario McMaster University Vancouver Island University Memorial University of Newfoundland Wilfrid Laurier University Ryerson University York University Saint Mary's University 1 Chile Adolfo Ibanez University University of Santiago Chile University of Chile Universidad Tecnica Federico Santa Maria Denmark Copenhagen Business School Technical University of Denmark -

Mathmatics and Sciences
MATHEMATICS SCIENCES Find out more about studying, research, life Studying in and work in the German Southwest Baden-Württemberg INTERNATIONAL DEGREE PROGRAMMES www.bw-studyguide.de [email protected] Follow us on www.facebook.com/bwstudyguide www.instagram.com/study_in_bw © 2019 Baden-Württemberg International | Photo: Baschi Bender / University of Freiburg Agricultural Economics (eng) 4 semesters University of Hohenheim www.uni-hohenheim.de/startseite.ht Bachelor Programmes ml?&L=1 Agricultural Sciences in the Tropics and 4 semesters University of Hohenheim www.uni-hohenheim.de/startseite.ht Subtropics (eng) ml?&L=1 Study Programme Standard Period Institution of Higher Education Web of Study Applied & Environmental Geoscience (eng) 4 semesters University of Tübingen www.uni-tuebingen.de/uni/qvr/e-30/ 30-02.html Biochemistry (eng, ger) 6 semesters University of Heidelberg www.uni-heidelberg.de/index_e.html Astro and Particle Physics (eng) 4 semesters University of Tübingen www.uni-tuebingen.de/uni/qvr/e-30/ Biological Sciences (eng, ger) 6 semesters University of Konstanz www.uni-konstanz.de/index.php?lang 30-02.html =en Biochemistry (eng) 4 semesters University of Tübingen www.uni-tuebingen.de/uni/qvr/e-30/ Biology (eng, ger) 6 semesters University of Heidelberg www.uni-heidelberg.de/index_e.html 30-02.html Biosciences (eng, ger) 6 semesters University of Heidelberg www.uni-heidelberg.de/index_e.html Biochemistry (eng, ger) 4 semesters University of Heidelberg www.uni-heidelberg.de/index_e.html Chemistry (eng, ger) 6 semesters University -

See the Final Program
2nd INTERNATIONAL SYMPOSIUM OF THE CRC 1052 “OBESITY MECHANISMS” September 2–4, 2019 in Leipzig Venue Mediencampus Villa Ida Poetenweg 28 04155 Leipzig phone +49 341-56296-704 The Mediencampus is an attractive meeting location in the center of Leipzig close to the Zoo. Highlights of the Meeting: - Outstanding research lectures in obesity - Poster exhibition - Get together with Blondie & The Brains (The band consists of members of the CRC 1052, legal musicians and the female singer) CRC 1052 Spokesperson Organizers Prof. Matthias Blüher Dr. Lysann Penkalla E-mail: [email protected] Leipzig University Phone: +49 341 97 22146 Faculty of Medicine Susanne Renno E-mail: [email protected] Clinic for Endocrinology and Phone: +49 341 97 13320 Nephrology This conference will be followed by the closing meeting of the Integrated Research and Treatment Center (IFB) AdiposityDiseases (September 3–4, 2019). Legend: PL Plenary lecture CRC-T CRC talk KL Keynote lecture IFB-T IFB talk PROGRAM Monday, 2nd September Tuesday, 3rd September Wednesday, 4th September 9:00 Session III IFB Symposium Adipose Tissue Heterogeneity Keynote Lecture 9:00-11:00 9:00-10:00 10:00 IFB Session II Arrival and Registration Psychosocial Aspects of 10:15-11:45 Obesity and Eating Disorder 10:00-11:00 11:00 Coffee Break Coffee Break 11:00-11:15 11:00-11:30 Session IV IFB Session III Lunch Time Snack Adipokines Highlights of the IFB in 12:00 11:45-12:45 11:15-13:00 Genetics and Neuroimaging Research Welcome 11:30-13:00 13:00 Session I Lunch -

2004-2005 Heidelberg College Catalog Heidelberg 310 East Market Street College Tiffin, Ohio 44883-2462 1.800.Heidelberg
Non-Profit Org. Heidelberg U.S. Postage PAID College Heidelberg 2004-2005 Heidelberg College Catalog Heidelberg 310 East Market Street College Tiffin, Ohio 44883-2462 1.800.Heidelberg www.heidelberg.edu 2004 - 2005 CATALOG Academic Year Calendar Introduction ' Semester I 2004-2005 1 Sun. Aug. 29 First-year students and transfers arrive Mon. Aug. 30 Registration verification Tues. Aug. 31 Classes begin Thur. Oct. 14 Long weekend recess begins after last class Mon. Oct. 18 Classes resume Tues. Nov. 23 Thanksgiving recess begins after last class Mon. Nov. 29 Classes resume Fri. Dec. 10 Classes end Mon. Dec. 13 Final exams begin Thur. Dec. 16 Christmas recess begins after last exam ' Semester II 2004-2005 Sun. Jan. 9 Registration verification Mon. Jan. 10 Classes begin Mon. Jan. 17 No classes—Martin Luther King Day Thur. Mar. 10 Spring recess begins after last class Tues. Mar. 29 Classes resume Thur. May 5 Classes end Fri. May 6 Final exams begin Wed. May 11 Final exams end Sun. May 15 Baccalaureate, Undergraduate and Graduate Commencement ' Summer 2005 Mon. May 23 Term 1 classes begin Fri. June 24 Term 1 classes end Mon. June 27 Term 2 classes begin Fri. July 29 Term 2 classes end Mon. May 23 Term 3 classes begin Fri. July 29 Term 3 classes end Sources of Information HEIDELBERG COLLEGE, Tiffin, Ohio 44883-2462 2 TELEPHONE SUBJECT OFFICE AREA CODE: 419 Admission Vice President for Enrollment 448-2330 Advanced Standing Vice President for Academic Affairs and Dean of the College 448-2216 Alumni Affairs Director of Alumni and Parent Relations