Melick & Porter, Llp Covid-19 Memo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UMBC Alumnae Racing to Develop Coronavirus Vaccine
Newsletter SPRING 2020 To our UMBC/Meyerhoff families: We hope you and your families are all doing well during this strange and stressful time of Covid- 19. Although the world has changed quickly with so many things shut down and many of us sheltering at home, we hope this newsletter will represent a ray of sunshine during a dark and difficult time. Please enjoy this positive representation of our student and alumni community. MPA Board UMBC Alumnae Racing to Develop Coronavirus Vaccine Kizzmekia Corbett ’08, M16, biological sciences, says it feels like she’s “living in a constant adrenaline rush.” Maybe that’s because she and her team at the Vaccine Research Center at the National Insti- tute of Allergy and Infectious Diseases have been working around the clock for weeks. They’re racing to develop a vaccine for the coronavirus faster than it can race across the globe. “To be living in this moment where I have the opportunity to work on something that has imminent global importance…it’s just a surre- al moment for me,” Corbett says. Despite it feeling surreal, the advances Corbett and her team are making are very real, and they’re setting records. “We are making better progress than I could have ever hoped for,” she says. After three months of studies in test tubes and in animals, the vaccine her team developed is about to enter a phase I clinical trial, a crucial hur- dle on the way to FDA approval. Read the complete article about Kizzmekia and her team’s efforts to develop a Covid-19 vaccine in the latest UMBC magazine at https:// Kizzmekia Corbett, NIH magazine.umbc.edu/umbc-alumnae-racing-to-develop- coronavirus-vaccine/. -

Officials Call on States to Expand Groups Getting COVID-19 Vaccines
Officials call on states to expand groups getting COVID-19 vaccines by Melissa Jenco, News Content Editor Editor's note:For the latest news on COVID-19, visit http://bit.ly/AAPNewsCOVID19. Federal health officials are releasing COVID-19 vaccine doses they had been withholding and are encouraging states to open vaccination to broader groups of people. "This next phase reflects the urgency of the situation we face," Health and Human Services Secretary Alex M. Azar II, J.D., said Tuesday. "Every vaccine dose that is sitting in a warehouse rather than going into an arm could mean one more life lost or one more hospital bed occupied." Azar said states should start vaccinating everyone 65 years and older and people ages 16 or 18 to 64 (depending on the vaccine) who have documented underlying conditions. The recommendation is a change from the initial recommendations on priority groups made by the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices last month. "We are telling states today they should open vaccination to all of their most vulnerable people," Azar said. "That is the most effective way to save lives now." Administration of vaccines from Pfizer-BioNTech and Moderna started in December among health care workers and residents of long-term care facilities, and roughly 9 million people have received at least one dose. However, that is significantly behind the 20 million officials predicted by the end of December. Azar blamed reporting lags and states micromanaging who could get a dose. He noted nearly 38 million doses, including 25 million first doses, have been distributed to states. -

Covid-19 Vaccines Are Coming
COVID-19 VACCINES ARE COMING. ARE WE READY? Etienne Grass – November 2020 On November 9, companies BioNTech and Pfizer 240 COVID vaccines are currently being developed announced the eagerly awaited preliminary results globally, according to the regular census carried out of their phase 3 clinical trial for a COVID vaccine by the World Health Organization. Of these, the project. While it is unusual for this type of result to WHO asserts 45 are in clinical trials2, although only be published before the trial is finalized, the rolling 35 according to the New York Times3. review procedure sanctioned by regulatory bodies to speed up the assessment of new drugs has The WHO says that 10 products are already tested enabled this transparency.1 in phase 3 , a figure that rises to 11 according to the New York Times, which includes the recent progress If the results have come as a positive surprise, it is of an Indian product in its assessment 4. mainly due to their high level of efficacy. The Food and Drug Administration (FDA) had indicated that it Within these phase 3 trials, four products are being would be ready to authorize a vaccine with more tested in China5 and one in Russia6. We do not have than 50% effectiveness. The first figures available the same level of information on them as on are much higher, at 90%. vaccines developed in the United States and Europe. For several weeks now, the head of the "Operation Technological platforms of current projects Warp Speed" program, Moncef Slaoui, who has been piloting American federal activities in support of Five products have in common that they are vaccine production since May, had been telling the integrated into the American (Operation Warp New York Times to be "confident that there will Speed-OWS), British and European partnerships7. -

Administration of Donald J. Trump, 2020 Remarks on Coronavirus
Administration of Donald J. Trump, 2020 Remarks on Coronavirus Vaccine Development and an Exchange With Reporters May 15, 2020 The President. Thank you very much. It's very hot today. Please, sit down. This is going to be a very hot one, and we apologize to everybody out there that's going to suffer through it. But you know what? It's better than bad weather. And it's great to be in the Rose Garden. I want to especially thank this group for joining us as we announce a historic, groundbreaking initiative in our ongoing effort to rapidly develop and manufacture a coronavirus vaccine. We're joined by Secretary Steven Mnuchin, Secretary Mark Esper, Secretary Alex Azar, Chairman of the Joint Chiefs of Staff General Mark Milley, FDA Director Dr. Stephen Hahn, Director of the National Institute of Health Dr. Francis Collins, Dr. Fauci, Dr. Birx. We're joined by a very terrific group of professionals. Tomorrow will mark 30 days since we released the White House guidelines for a safe and phased opening of America. That's what we're doing. It's the opening of America. We're going to have an amazing year next year. We're going to have a great transition into the fourth quarter. As of this morning, almost every State has taken steps to begin reopening, and the American people are doing an extraordinary job of continuing to take precautions while, at the same time, wanting to start—and they will be starting—to resume their American way of life. We will be reigniting our economic engines. -

April 23, 2020
April 23, 2020 Greetings National Dental Association Members! It has been almost 6 weeks since the COVID-19 pandemic disrupted our daily lives. We will forever make references to Pre-COVID-19 practices and Post-COVID-19 practices. Although we are not out of the woods with regard to Coronavirus, it does appear that we may be starting to see some slight “flattening of the proverbial curve” that is tracking the Coronavirus and its effects in the United States. The National Dental Association Coronavirus Task Force has been working every single day since the inception of this pandemic, gathering information to provide for you, so that you will be able to navigate with ease during this time, assist your Dr. Sheila L. Armstrong community and return to work safely. Over these next few weeks, we are going to see the Governors start to “re-open” the states and allow certain services and businesses to resume operation. Due to the fact that we as dentist work in highly contagious environments, we must be prepared to ensure that every visit to the dental office is safe for the patient, the staff, and most importantly you the dentist. It will not be business as usual or Pre-COVID-19 practices, we will be going to the next level in everything that we do, from infection control, patient encounter management, and being recognized as a source for providing screening and testing for COVID-19. The National Dental Association is committed to bringing you the most up-to date information regarding financial assistance during this unprecedented time in our history. -

March 11, 2021
Sector Email 3.11.2021 Subject header: Updates on COVID-19 from Public Health - Seattle & King County (PHSKC) Schools Dear school partners, March is Women’s History Month and this year we are grateful to some of the women who are making history by changing the course of the COVID-19 pandemic. Have you learned about Kizzmekia Corbett, an African American Immunologist who co-led the development of the Moderna COVID-19 vaccine. This month, we celebrate Dr. Corbett and thousands of her colleagues in healthcare, research and public health as women who are making history. Reminder: please review the King County Schools COVID-19 Response Toolkit, related resources, and training videos. ---------- This week’s Public Health—Seattle & King County (PHSKC) Schools and Child Care Task Force sector email includes the following topics: 1. Key indicators of COVID-19 activity 2. Vaccine Updates a. Educators, school staff and child care workers now eligible for COVID-19 vaccine b. How school and child care staff can access vaccines c. Getting Vaccinated Resources d. Signs and Symptoms Following Vaccination 3. COVID Community Vaccination Event Planning Workbooks 4. Special Enrollment for Washington Health Care 5. Updated Mask Posters 6. Events a. Public Health’s upcoming trainings and community discussions on COVID-19 b. COVID-19 Vaccination Webinar and Q&A c. COVID-19 Vaccine Distribution Webinar and Q&A d. Oromo Session – COVID-19 and Vaccinations e. Somali Session – COVID-19 and Vaccinations 7. Updated Guidance from the CDC – When You’ve Been Fully Vaccinated 8. School and District Town Hall Meetings 9. -

May 5, 2021 COVID+HIV Update
SELECT LANGUAGE DONATE SEARCH ABOUT GROUPS YOUTH SERVICES COVID-19 RESOURCES UPDATES EVENTS GET INVOLVED Home / COVID-19 / COVID updates / COVID-19 and HIV updates COVID-19 AND HIV UPDATES MAY 5, 2021 SIGN UP FOR OUR NEWSLETTER HERE Below are this week’s East Bay COVID-19 and HIV updates. This page is usually updated on Wednesday evenings with data and resources gathered from many collaborators in Alameda County, Contra Costa County, Solano County, CA state. Please click here to share feedback. VACCINES MASKS GUIDANCE RESOURCES ARCHIVES PDF SUMMARY The SARS-CoV-2 virus Jump to: (NIAID) Key East Bay COVID-19 updates Vaccine access; updates on the J&J and other vaccines; vaccines for people living with HIV Disparities data and studies Harm reduction: prevention for vaccinated people and masks HIV updates Jobs, funding, training opportunities and other resources COVID testing and other top links EAST BAY COVID-19 UPDATES Everyone ages 16 and over in the US is eligible for a free COVID-19 vaccine, regardless of insurance and documentation status. Vaccine supply in the East Bay is now plentiful for the three authorized vaccines: P됍zer, Moderna and Johnson & Johnson. Appointments are available the same day at MyTurn.ca.gov, including the P됍zer vaccine for 16-17-year-olds. Click here for more on vaccine eligibility and how to get one. The FDA is expected to authorize the P됍zer vaccine for 12–15-year-olds in the coming week. P됍zer plans to submit authorization requests for children ages 2-11 in September. Moderna has been studying its vaccine in children ages 6 months to 18 years and is also expected to release some results soon. -

People in the News
Volume XXX, Number XLII December 24, 2020 - January 6, 2021 Seeking to end the ‘Slavery Loophole’ - See Page 3 Black nurse takes the first vaccine Visit us online at www.northdallasgazette.com - See Page 4 COVID-19 won’t stop toy giveaway - See Page 5 Advocating for education in Washington, DC - See Page 6 Black women fill top posts under Biden - See Page 8 Cowboys stun the 49ers - See Page 9 A look back at the best films of 2020 - See Page 11 Sister Tarpley: New Year, New Attitude Background: OC Gonzalez / Unsplash, Pride: Republic Country Club / Wikimedia, Harris: Official Portrait, OC Gonzalez / Unsplash, Pride: Republic Country Club Wikimedia, Background: - See Page 15 Inside... See Page 2 People In The News ... People in the News ........................................ 2 Op/Ed ............................................................ 3 Health ............................................................ 4 Community .................................................... 5 Education ...................................................... 6 Features ........................................................ 8 Dallas Cowboys ............................................. 9 NDG Bookshelf ............................................ 11 Dr. Kizzmekia Corbett Gen. Lloyd Austin Rashida Jones Marketplace ................................................ 12 Career / Notices .......................................... 13 NDG Quote of the Week: ““The future belongs to those who prepare for it today.” Church Directory ................................... -

The COVID-19 Wicked Problem in Public Health Ethics: Conflicting
COMMENT https://doi.org/10.1057/s41599-021-00839-1 OPEN The COVID-19 wicked problem in public health ethics: conflicting evidence, or incommensurable values? ✉ Federica Angeli 1 , Silvia Camporesi 2 & Giorgia Dal Fabbro3 While the world was facing a rapidly progressing COVID-19 second wave, a policy paradox emerged. On the one side, much more was known by Autumn 1234567890():,; 2020 about the mechanisms underpinning the spread and lethality of Sars-CoV- 2. On the other side, how such knowledge should be translated by policymakers into containment measures appeared to be much more controversial and debated than during the first wave in Spring. Value-laden, conflicting views in the scientific community emerged about both problem definition and subsequent solutions surrounding the epidemiological emergency, which underlined that the COVID-19 global crisis had evolved towards a full-fledged policy “wicked pro- blem”. With the aim to make sense of the seemingly paradoxical scientific dis- agreement around COVID-19 public health policies, we offer an ethical analysis of the scientific views encapsulated in the Great Barrington Declaration and of the John Snow Memorandum, two scientific petitions that appeared in October 2020. We show that how evidence is interpreted and translated into polar opposite advice with respect to COVID-19 containment policies depends on a different ethical compass that leads to different prioritization decisions of ethical values and societal goals. We then highlight the need for a situated approach to public health policy, which recognizes that policies are necessarily value-laden, and need to be sensitive to context-specific and historic socio-cultural and socio- economic nuances. -

Black Scientist Suggests COVID-19 Is a 'Genocide' Against Blacks
4/21/2020 Black Scientist Suggests COVID-19 Is a 'Genocide' Against Blacks, Department of Health Launches Investigation Into Her Tweets Black Scientist Suggests COVID-19 Is a ‘Genocide’ Against Blacks, Department of Health Launches Investigation Into Her Tweets By Nefeteria Brewster - April 20, 2020 The Department of Health and Human Services is investigating a controversial series of tweets posted by a Black scientist who has been a vital researcher in the race to create a vaccine for COVID-19, Fox News reported. Many know Dr. Kizzmekia Corbett, a viral immunologist working with the National Institute of Allergy and Infectious Diseases, as a lead researcher with Dr. Barney Graham’s coronavirus team in the Vaccine Research Center, which is part of the National Institutes of Health. https://atlantablackstar.com/2020/04/20/black-scientist-suggests-covid-19-is-a-genocide-against-blacks-department-of-health-launches-investigation-into-her-tweets/ 1/7 4/21/2020 Black Scientist Suggests COVID-19 Is a 'Genocide' Against Blacks, Department of Health Launches Investigation Into Her Tweets Kizzmekia Corbett is a research fellow at the NIH Vaccine Research Center and a key member of a team helping to develop a vaccine for the COVID-19 virus. (Photo: UNC School of Medicine) Recently Corbett has been drawing public attention because of her social media presence, particularly several controversial tweets on her Twitter page. Some of the tweets date back to the beginning of February. On March 29 Corbett tweeted a link to a Bloomberg article about the impact of the virus on poor communities. In her accompanying comments, she suggested that doctors may choose to overlook Blacks if ventilators are in short supply. -

Covid-19: Group of UK and US Experts Argues
NEWS BMJ: first published as 10.1136/bmj.m3908 on 7 October 2020. Downloaded from New York Cite this as: BMJ 2020;371:m3908 Covid-19: Group of UK and US experts argues for “focused protection” http://dx.doi.org/10.1136/bmj.m3908 instead of lockdowns Published: 07 October 2020 Jeanne Lenzer Thousands of medical practitioners and public health Benefits and harms scientists have signed a declaration arguing for an On Monday the declaration’s authors met with Alex alternative public health approach to dealing with Azar, US secretary of health and human services, and covid-19. Scott Atlas, an adviser to President Trump, for what The Great Barrington Declaration,1 published on Kulldorff described as a “very good discussion.”6 Monday 5 October, was drawn up by three However, Stefan Baral, a physician epidemiologist epidemiologists and public health experts from and associate professor at Johns Hopkins University, Harvard, Oxford, and Stanford universities, who said he was concerned that the meeting had taken describe their approach as “focused protection” of place. Baral explained that, while he generally agreed the people most at risk. that lockdowns were causing serious harms, he had As of Wednesday 7 October almost 6300 medical declined to sign the declaration because it did not practitioners and public health scientists from the address the concrete steps needed to assist the most US, the UK, and other nations had signed the vulnerable people. declaration. Baral told The BMJ that three steps must accompany The authors—Martin Kulldorff, professor of medicine any loosening of restrictions: firstly, the removal of at Harvard, Sunetra Gupta, professor of theoretical any barriers to accessing healthcare; secondly, paid epidemiology at Oxford, and Jay Bhattacharya, leave for people affected by covid-19; and lastly, professor of medicine and economics at housing support for such people in multigenerational Stanford—said that because older people were 1000 households. -
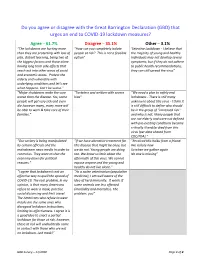
Do You Agree Or Disagree with the Great Barrington Declaration (GBD
Do you agree or disagree with the Great Barrington Declaration (GBD) that urges an end to COVID-19 lockdown measures? Agree – 61.7% Disagree – 35.1% Other – 3.1% “The lockdowns are hurting more “How can you completely isolate ‘Selective Lockdown - I believe that than they are protecting with loss of people at risk? This is not a feasible the majority of young and healthy jobs, distant learning, being two of option” individuals may not develop severe the biggest factors and those alone symptoms; but if they do not adhere having long term side effects that to public health recommendations, reach out into other areas of social they can still spread the virus” and economic areas. Protect the elderly and vulnerable with underlying conditions and let’s see what happens. Can't be worse.” “Major shutdowns make the cure “Tasteless and written with severe “We need a plan to safely end worse than the disease. Yes, some bias” lockdowns - There is still many people will get very sick and even unknowns about this virus - I think it die however many, many more will is still difficult to define who should be able to work & take care of their be in the group of "increased risk" families.” and who is not. Many people that are not elderly and were not defined with pre-existing conditions became critically ill and/or died from this virus (per data shared from CDC/FDA).” “Our society is being manipulated “If we have alterative treatment for "Received this haiku from a friend: by certain officials and the this disease that might be okay, but We isolate now mainstream news media in order to we do not.