Laparoscopic Hiatal Hernia Repair in Patients with an Intrathoracic Pancreas: Case Series and a Review of Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PYLORIC STENOSIS of INFANCY-PERSPECTIVES Different Views from Different Bridges
Central Annals of Pediatrics & Child Health Perspective *Corresponding author IAN M. ROGERS, Department of Surgery, AIMST University, 46 Whitburn Road, Cleadon, Tyne and Wear, SR67QS, Kedah, Malaysia, Tel: 01915367944; PYLORIC STENOSIS OF INFANCY- 07772967160; Email: [email protected] Submitted: 18 March 2020 PERSPECTIVES Different views Accepted: 27 March 2020 Published: 30 March 2020 ISSN: 2373-9312 from different bridges Copyright © 2020 ROGERS IM IAN M ROGERS* OPEN ACCESS Department of Surgery, AIMST University, Malaysia Keywords Abstract • Neonatal hyperacidity; Neonatal hypergastrinaemia; Baby and parents perspectives of diagnosis Introduction: The differing experiences of the surgeon, he parent and the baby and treatment; Spectrum of presentation of are discussed in terms of what is presently known about the pathogenesis of pyloric pyloric stenosis of infancy; Primary hyperacidity stenosis of infancy (PS). pathogenesis of pyloric stenosis Methods: The experiences are culled from personal experience and from the comments made on online pressure and support groups for pyloric stenosis of infancy. These comments are reviewed from the standpoint of the Primary Hyperacidity pathogenesis of cause. Conclusion: The present perspectives of the PS surgeon and the difficulties experienced by the PS family fit well which the basic tenets of the Primary Hyperacidity theory. Reference to this theory allows a satisfactory explanation for difficulties in diagnosis and for the variability of presentation. INTRODUCTION In 1961 this theme was further developed by Dr Jacob. He again recognised the milder forms-those in which a long- term The Surgeon cure could similarly be achieved without surgery. A 1% mortality Everybody knows: For progressive pyloric stenosis of infancy in 100 patients was achieved with the usual medical measures (PS), pyloromyotomy reigns supreme! Impending catastrophe of which a reduced feeding regime was judged to be especially transformed safely, in a few minutes, into an enduring cure. -

Massive Hiatal Hernia Involving Prolapse Of
Tomida et al. Surgical Case Reports (2020) 6:11 https://doi.org/10.1186/s40792-020-0773-8 CASE REPORT Open Access Massive hiatal hernia involving prolapse of the entire stomach and pancreas resulting in pancreatitis and bile duct dilatation: a case report Hidenori Tomida* , Masahiro Hayashi and Shinichi Hashimoto Abstract Background: Hiatal hernia is defined by the permanent or intermittent prolapse of any abdominal structure into the chest through the diaphragmatic esophageal hiatus. Prolapse of the stomach, intestine, transverse colon, and spleen is relatively common, but herniation of the pancreas is a rare condition. We describe a case of acute pancreatitis and bile duct dilatation secondary to a massive hiatal hernia of pancreatic body and tail. Case presentation: An 86-year-old woman with hiatal hernia who complained of epigastric pain and vomiting was admitted to our hospital. Blood tests revealed a hyperamylasemia and abnormal liver function test. Computed tomography revealed prolapse of the massive hiatal hernia, containing the stomach and pancreatic body and tail, with peripancreatic fluid in the posterior mediastinal space as a sequel to pancreatitis. In addition, intrahepatic and extrahepatic bile ducts were seen to be dilated and deformed. After conservative treatment for pancreatitis, an elective operation was performed. There was a strong adhesion between the hernial sac and the right diaphragmatic crus. After the stomach and pancreas were pulled into the abdominal cavity, the hiatal orifice was closed by silk thread sutures (primary repair), and the mesh was fixed in front of the hernial orifice. Toupet fundoplication and intraoperative endoscopy were performed. The patient had an uneventful postoperative course post-procedure. -

CHAPTER 8 – DIGESTIVE SYSTEM OBJECTIVES on Completion of This Chapter, You Will Be Able To: • Describe the Digestive System
CHAPTER 8 – DIGESTIVE SYSTEM OBJECTIVES On completion of this chapter, you will be able to: • Describe the digestive system. • Describe the primary organs of the digestive system and state their functions. • Describe the two sets of teeth with which humans are provided. • Describe the three main portions of a tooth. • Describe the accessory organs of the digestive system and their functions. • Describe digestive differences of the child and older adult. • Analyze, build, spell, and pronounce medical words. • Comprehend drugs highlighted in this chapter • Describe diagnostic and laboratory tests related to the digestive system • Identify and define selected abbreviations. • Describe each of the conditions presented in the Pathology Spotlights. • Complete the Pathology Checkpoint. • Complete the Study and Review section and the Chart Note Analysis. OUTLINE I. Anatomy and Physiology Overview (Fig. 8–1, p. 189) Also known as the alimentary canal and/or gastrointestinal tract, this continuous tract begins with the mouth and ends with the anus. Consist of primary and accessory organs that convert food and fluids into a semiliquid that can be absorbed for use by the body. Three main functions of the digestive system are: 1. Digestion – the process by which food is changed in the mouth, stomach, and intestines by chemical, mechanical and physical action, so that it can be absorbed by the body. 2. Absorption –the process whereby nutrient material is taken into the bloodstream or lymph and travels to all cells of the body. 3. Elimination –the process whereby the solid products of digestion are excreted. A. Mouth (Fig. 8–2, p. 190) – a cavity formed by the palate, tongue, lips, and cheeks. -

A Rare Case of Pancreatitis from Pancreatic Herniation
Case Report J Med Cases. 2018;9(5):154-156 A Rare Case of Pancreatitis From Pancreatic Herniation David Doa, c, Steven Mudrocha, Patrick Chena, Rajan Prakashb, Padmini Krishnamurthyb Abstract nia sac, resulting in mediastinal abscess which was drained sur- gically. He had a prolonged post-operative recovery following Acute pancreatitis is a commonly encountered condition, and proper the surgery. In addition, he had a remote history of exploratory workup requires evaluation for underlying causes such as gallstones, laparotomy for a retroperitoneal bleed. He did not have history alcohol, hypertriglyceridemia, trauma, and medications. We present a of alcohol abuse. His past medical history was significant for case of pancreatitis due to a rare etiology: pancreatic herniation in the hypertension and osteoarthritis. context of a type IV hiatal hernia which involves displacement of the On examination, his vitals showed a heart rate of 106 stomach with other abdominal organs into the thoracic cavity. beats/min, blood pressure of 137/85 mm Hg, temperature of 37.2 °C, and respiratory rate of 20/min. Physical exam dem- Keywords: Pancreatitis; Herniation; Hiatal hernia onstrated left-upper quadrant tenderness without guarding or rebound tenderness, with normoactive bowel sounds. Lipase was elevated at 2,687 U/L (reference range: 73 - 383 U/L). His lipase with the first episode of abdominal pain had been 1,051 U/L. Hematocrit was 41.2% (reference range: 42-52%), Introduction white cell count was 7.1 t/mm (reference range 4.8 - 10.8 t/ mm) and creatinine was 1.0 mg/dL (references range: 0.5 - 1.4 Acute pancreatitis is a commonly encountered condition with mg/dL). -

A Case Report
J Pediatr Adolesc Surg. 2020; 1:89-91 OPEN ACCESS DOI: https://doi.org/10.46831/jpas.v1i2.39 Case Report Postoperative gastric Outlet Obstruction following hiatal hernia repair in an infant: A case report Muhammad Jawad Afzal,1 Shabbir Ahmad,1 Farrakh Mehmood Satar,1 Sajid Iqbal Nayyer,2 Muhammad Bilal Mirza,2 Nabila Talat1 Department of Pediatric Surgery II, The Children’s Hospital and the Institute of Child Health, Lahore Cite as: Afzal MJ, Ahmad S, Satar FM, Nayyer SI, Mirza MB, Talat N. Postoperative gastric Outlet Obstruction following hiatal hernia repair in an infant: A case report J Pediatr Adolesc Surg. 2020; 1: 89-91. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited (https://creativecommons.org/ licenses/by/4.0/). ABSTRACT Background: Infantile hypertrophic pyloric stenosis (IHPS) is an exceedingly rare cause of postoperative emesis in a case of hiatal hernia. Occasionally it may simulate other etiology of gastric outlet obstruction. Case Presentation: A 32-day-old male baby presented with respiratory distress and vomiting since birth. Diagnosis of eventra- tion of left hemi diaphragm was made on CT Chest. At surgery, hiatal hernia with an intrathoracic stomach was found, which was repaired. On 5th postoperative day, the baby developed vomiting after feeding which gradually turned to be projectile in nature over a week. Contrast meal performed showed malpositioned stomach with delayed emptying. At re-operation, a well- formed olive of pylorus was encountered; Ramstedt pyloromyotomy was done. -

Type IV Paraesophageal Hiatal Hernia and Organoaxial Gastric Volvulus
Copyright © 2012 by MJM MJM 2012 14(1): 7-12 7 CASE REPORT Type IV paraesophageal hiatal hernia and organoaxial gastric volvulus Alison Zachry, Alan Liu, Sabiya Raja, Nadeem Maboud, Jyotu Sandhu, DeAndrea Sims, Umer Feroze Malik*, Ahmed Mahmoud. ABSTRACT: Organoaxial gastric volvulus occurs when the stomach rotates on its longitudinal axis connecting the gastroesophageal junction to the pylorus. With that, the antrum of the stomach usually rotates in the opposite direction in rela- tion to its fundus (1). This phenomenon has often been known to be associated with diaphragmatic defects (2) Importantly, abnormal rotation of the stomach of more than 180° is a life threatening emergency that may create a closed loop ob- struction which may result in incarceration leading to strangulation, and hence, a surgical emergency. We present the case of a middle-aged female who presented with organoaxial gastric volvulus and had an associated Type IV paraesophageal hiatal hernia that was treated electively. Normally an emergent gastric volvulus is diagnosed via Borchardt’s classic triad (epigastric pain, unproductive vomiting and diffculty inserting a nasogastric tube); however in this patient the nasogastric tube (NGT) was passed into the antrum which allowed additional time for resusci- tation with fuids and other symptomatic relief. CASE REPORT a systemic review was negative. Abdominal A 56 year old Caucasian female presented examination revealed hyperactive bowel sounds. with inability to eat or drink due to persistent nausea The patient’s abdomen was mildly distended in and small amount of non bilious, non bloody emesis. the epigastric region. Her abdomen was soft with The patient reported no bowel movements and an tenderness upon palpation in both the left upper inability to pass fatus for one week. -

Pathophysiology of Hiatal Hernia
Otterbein University Digital Commons @ Otterbein Nursing Student Class Projects (Formerly MSN) Student Research & Creative Work 2017 Pathophysiology of Hiatal Hernia Courtney Adams [email protected] Follow this and additional works at: https://digitalcommons.otterbein.edu/stu_msn Part of the Nursing Commons Recommended Citation Adams, Courtney, "Pathophysiology of Hiatal Hernia" (2017). Nursing Student Class Projects (Formerly MSN). 252. https://digitalcommons.otterbein.edu/stu_msn/252 This Project is brought to you for free and open access by the Student Research & Creative Work at Digital Commons @ Otterbein. It has been accepted for inclusion in Nursing Student Class Projects (Formerly MSN) by an authorized administrator of Digital Commons @ Otterbein. For more information, please contact [email protected]. Courtney M. Adams RN, BSN, CCRN Otterbein University, Westerville, Ohio Akst, L. M., Haque, O. J., Clarke, J. O., Hillel, • A. T., Best, S. R.A, and Altman, K. W. (2017). • I was diagnosed with a hiatal hernia from a Patients are diagnosed by endoscopy, barium swallow, esophageal The changing impact of gastroesophageal • manometry and high-resolution manometry (HRM) post GERD reflux disease in clinical practice: An updated car accident causing an everyday symptom GERD being the main symptom of HH requires us to know the • Increased pressure from the abdominal cavity symptoms. Many studies are favoring HRM with ruling out the study. Annals of Otology, Rhinology, & of gastroesophageal reflux disease (GERD). pathophysiology of GERD to know how to manage a HH. Laryngology, 126(3), 229-235. can cause the herniation of contents from • The lower esophageal sphincter may cause GERD by the muscle diagnosis of HH (Khajanchee, Cassera, Swanstrom, & Dunst, 2013). -

Hiatal Hernia by Mayo Clinic Staff
Reprints A single copy of this article may be reprinted for personal, noncommercial use only. Hiatal hernia By Mayo Clinic staff Original Article: http://www.mayoclinic.com/health/hiatal-hernia/DS00099 Definition A hiatal hernia occurs when part of your stomach pushes upward Hiatal hernia through your diaphragm. Your diaphragm normally has a small opening (hiatus) through which your food tube (esophagus) passes on its way to connect to your stomach. The stomach can push up through this opening and cause a hiatal hernia. In most cases, a small hiatal hernia doesn't cause problems, and you may never know you have a hiatal hernia unless your doctor discovers it when checking for another condition. But a large hiatal hernia can allow food and acid to back up into your esophagus, leading to heartburn. Self-care measures or medications can usually relieve these symptoms, although a very large hiatal hernia sometimes requires surgery. Symptoms Small hiatal hernias Most small hiatal hernias cause no signs or symptoms. Large hiatal hernias Larger hiatal hernias can cause signs and symptoms such as: • Heartburn • Belching • Difficulty swallowing • Fatigue When to see a doctor Make an appointment with your doctor if you have any persistent signs or symptoms that worry you. Causes A hiatal hernia occurs when weakened muscle tissue allows Hiatal hernia your stomach to bulge up through your diaphragm. It's not always clear why this happens, but pressure on your stomach may contribute to the formation of hiatal hernia. How a hiatal hernia forms Your diaphragm is a large dome-shaped muscle that separates your chest cavity from your abdomen. -

Rationale and Results of Anatomic Repair of Esophageal Hiatus Hernia Conrad R
Henry Ford Hospital Medical Journal Volume 24 | Number 4 Article 7 12-1976 Rationale and results of anatomic repair of esophageal hiatus hernia Conrad R. Lam Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Lam, Conrad R. (1976) "Rationale and results of anatomic repair of esophageal hiatus hernia," Henry Ford Hospital Medical Journal : Vol. 24 : No. 4 , 243-254. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol24/iss4/7 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp Med Journal Vol 24, No 4,1976 Rationale and results of anatomic repair of esophageal hiatus hernia* Conrad R. Lam, M.D.** A logical method of repair of esophageal /\ logical method for the repair of es- hiatus hernia based on anatomic principles phageal hiatus hernia based on anatomic was proposed nearly 25 years ago by the late principles was proposed nearly 25 years ago Phillip Allison. There was general agreement by the late Philip Allison, then of Leeds and that his operation had advantages, but be later of Oxford, England, in his paper titled cause of a disappointing number of recur "Reflux Esophagitis, Sliding Hernia and the rences and problems with reflux, more Anatomy of Repair."^ He correctly stressed complicated operations were devised. Some the fact that reflux esophagitis is a complica- of these involved plication of the stomach tion of sliding hernia and not paraesopha around the lower esophagus (Nissen, geal hernia, for which he coined the term Belsey). -

Hiatal Hernia
FACT SHEET FOR PATIENTS AND FAMILIES Hiatal Hernia What is it? Normal esophagus A hiatal hernia is a condition in which the top of the stomach bulges through an opening in the diaphragm called the hiatus. The diaphragm is the large muscle The diaphragm The hiatus is that helps you breathe. The diaphragm separates the is a muscle that an opening in stomach from the chest. A hiatal hernia moves up into separates the chest the diaphragm and abdomen that lets the the chest and can cause pain and other symptoms. esophagus reach the stomach There are 2 types of hiatal hernias: • A sliding hernia is the most common. The top part of the stomach and the part of the tube that connects Hiatal hernias your mouth to your stomach (the esophagus) slide up into the chest through the hiatus. • A paraesophageal hernia is less common. A lower part of the stomach squeezes through the hiatus and gets stuck next to the esophagus. This is more serious because it can cut off the blood supply to that part of the stomach. What causes it and who is at risk? With a sliding hernia, the With a paraesophageal top of the stomach pushes hernia, a lower section The exact causes are not known, but it’s a common through the diaphragm of the stomach pushes condition in adults. As many as 10 out of 100 people through the diaphragm who are over age 40 have a hiatal hernia. The chances for getting it increase as a person gets older. What are the symptoms? Other things that increase your chances for getting Most small hiatal hernias don’t cause symptoms and hiatal hernia include: are never discovered. -
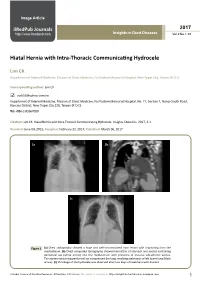
Hiatal Hernia with Intra-Thoracic Communicating Hydrocele
Image Article iMedPub Journals 2017 http://www.imedpub.com Insights in Chest Diseases Vol. 2 No. 1: 30 Hiatal Hernia with Intra-Thoracic Communicating Hydrocele Lim CK Department of Internal Medicine, Division of Chest Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan (R.O.C) Corresponding author: Lim CK [email protected] Department of Internal Medicine, Division of Chest Medicine, Far Eastern Memorial Hospital, No. 21, Section 2, Nanya South Road, Banciao District, New Taipei City 220, Taiwan (R.O.C). Tel: +886-2-89667000 Citation: Lim CK. Hiatal Hernia with Intra-Thoracic Communicating Hydrocele. Insights Chest Dis. 2017, 2:1. Received: June 04, 2015; Accepted: February 23, 2017; Published: March 06, 2017 1a 1b 1c Figure 1 (a) Chest radiography showed a huge and well-circumscribed mass lesion with originating from the mediastinum. (b) Chest computed tomography showed herniation of stomach and ascites-containing peritoneal sac (white arrow) into the mediastinum with presence of massive sub-phrenic ascites. The ascites-containing peritoneal sac compressed the lung, resulting atelectasis of left lower lung (black arrow). (c) Shrinkage of the hydrocele was observed after two days of treatment with diuretic. © Under License of Creative Commons Attribution 3.0 License | This article is available in: http://insightsinchestdiseases.imedpub.com 1 ARCHIVOS DE MEDICINA Insights in Chest Diseases 2017 ISSN 1698-9465 Vol. 2 No. 1: 30 Clinical Study outpatient thereafter for alcoholic liver cirrhosis. He also receives furosemide and spironolactone regularly for ascites control. No A 72-year-old man with alcoholic liver cirrhosis presented to recurrence of the intra-thoracic communicating hydrocele since our clinic with the chief complaints of shortness of breath and then. -

Hiatal Hernia
® ® GastroenteroloGy department Hiatal Hernia definition causes A hernia occurs when one part of the body protrudes through The exact cause of hiatal hernias isn’t known. Your chest cavity a gap or opening into another part. A hiatal hernia forms at the and abdomen are separated by your diaphragm — a large opening in your diaphragm where your food pipe (esophagus) dome-shaped muscle that’s responsible for a major part of joins your stomach. Part of the stomach pushes through this normal breathing. Your esophagus passes into your stomach opening causing a hiatal hernia. through an opening in the diaphragm called the hiatus. Hiatal hernias occur when the muscle tissue surrounding this opening Most small hiatal hernias don’t cause problems, and you may becomes weak, and the upper part of your stomach bulges up never know you have a hiatal hernia unless your doctor discovers through the diaphragm into your chest cavity. it when checking for another condition. But a large hiatal hernia can allow food and acid to back up into your esophagus, leading pressure on the abdomen to heartburn and chest pain. Self-care measures or medications can usually relieve these symptoms, although very large hiatal Some people develop a hiatal hernia after an injury to the area. hernias sometimes need surgical repair. Others are born with an inherent weakness or unusually large hiatal opening. But anything that puts intense pressure on your symptoms abdomen — including persistent or severe coughing or vomiting, pregnancy, straining while going to the bathroom, increased Small hernias — Most small hiatal hernias cause no problems.