Baseline Report Series:1. the Triassic Sandstones of the Vale of York
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Building Stone Atlas of Warwickshire
Strategic Stone Study A Building Stone Atlas of Warwickshire First published by English Heritage May 2011 Rebranded by Historic England December 2017 Introduction The landscape in the county is clearly dictated by the Cob was suitable for small houses but when more space was underlying geology which has also had a major influence on needed it became necessary to build a wooden frame and use the choice of building stones available for use in the past. The wattle fencing daubed with mud as the infilling or ‘nogging’ to geological map shows that much of this generally low-lying make the walls. In nearly all surviving examples the wooden county is underlain by the red mudstones of the Triassic Mercia frame was built on a low plinth wall of whatever stone was Mudstone Group. This surface cover is however, broken in the available locally. In many cases this is the only indication we Nuneaton-Coventry-Warwick area by a narrow strip of ancient have of the early use of local stones. Adding the stone wall rocks forming the Nuneaton inlier (Precambrian to early served to protect the wooden structure from rising damp. The Devonian) and the wider exposure of the unconformably infilling material has often been replaced later with more overlying beds of the Warwickshire Coalfield (Upper durable brickwork or stone. Sometimes, as fashion or necessity Carboniferous to early Permian). In the south and east of the dictated, the original timber framed walls were encased in county a series of low-lying ridges are developed marking the stone or brick cladding, especially at the front of the building outcrops of the Lower and Middle Jurassic limestone/ where it was presumably a feature to be admired. -

3-D Bedrock Geology Model of the Permo-Triassic of Yorkshire and East Midlands
3-D Bedrock geology model of the Permo-Triassic of Yorkshire and East Midlands Geology and Landscape Southern Britain Programme Internal Report CR/06/091 BRITISH GEOLOGICAL SURVEY GEOLOGY AND LANDSCAPE SOUTHERN BRITAIN PROGRAMME INTERNAL REPORT CR/06/091 The National Grid and other Ordnance Survey data are used with the permission of the 3-D Bedrock geology model of Controller of Her Majesty’s Stationery Office. Licence No: 100017897/2006. the Permo-Triassic of Yorkshire Keywords and East Midlands Report; 3-D Model; Sherwood Sandstone Group; Mercia Mudstone Group; Sneinton Formation; Permian; Roxby Formation; Brotherton Ford, J., Napier, B., Cooper, A., Pharaoh, T., Vincent, C., Carney, Formation; Edlington Formation; J., Thorpe, S., Brayson, J. Cadeby Formation; Yellow Sands Formation; Basal Permian Breccia; Aquifer;. Front cover Model output showing faulted elevation grid for the base Sherwood Sandstone Group and the thickness of the Sherwood Sandstone Group Bibliographical reference FORD, J., NAPIER, B., COOPER, A., PHARAOH, T., VINCENT, C., CARNEY, J., THORPE, S., BRAYSON, J.. 2005. 3-D Bedrock geology model of the Permo-Triassic of Yorkshire and East Midlands. British Geological Survey Internal Report, CR/06/091. 39pp. Copyright in materials derived from the British Geological Survey’s work is owned by the Natural Environment Research Council (NERC) and/or the authority that commissioned the work. You may not copy or adapt this publication without first obtaining permission. Contact the BGS Intellectual Property Rights Section, British Geological Survey, Keyworth, e-mail [email protected]. You may quote extracts of a reasonable length without prior permission, provided a full acknowledgement is given of the source of the extract. -

Developing a Geological Framework
21/2/12 GeolFrameworkPaper_postreview_v2acceptchanges_editorcomments New insights from 3D geological models at analogue CO2 storage sites in Lincolnshire and eastern Scotland, UK. Alison Monaghan1*, Jonathan Ford2, Antoni Milodowski2, David McInroy1, Timothy Pharaoh2, Jeremy Rushton2, Mike Browne1, Anthony Cooper2, Andrew Hulbert2 and Bruce Napier2 1 British Geological Survey, Murchison House, West Mains Road, Edinburgh, EH9 3LA, UK. 2 British Geological Survey, Kingsley Dunham Centre, Keyworth, Nottingham, NG12 5GG, UK. * Corresponding author (email [email protected] (Approx.15,600 words in total, 25 figures) SUMMARY: Subsurface 3D geological models of aquifer and seal rock systems from two contrasting analogue sites have been created as the first step in an investigation into methodologies for geological storage of carbon dioxide in saline aquifers. Development of the models illustrates the utility of an integrated approach using digital techniques and expert geological knowledge to further geological understanding. The models visualize a faulted, gently dipping Permo-Triassic succession in Lincolnshire and a complex faulted and folded Devono-Carboniferous succession in eastern Scotland. The Permo-Triassic is present in the Lincolnshire model to depths of -2 km OD, and includes the aquifers of the Sherwood Sandstone and Rotliegendes groups. Model-derived thickness maps test and refine Permian palaeogeography, such as the location of a carbonate reef and its associated seaward slope, and the identification of aeolian dunes. Analysis of borehole core samples established average 2D porosity values for the Rotliegendes (16%) and Sherwood Sandstone (20%) groups, and the Zechstein (5%) and Mercia Mudstone (<10%) groups, which are favourable for aquifer and seal units respectively. Core sample analysis has revealed a complex but well understood diagenetic history. -

Geological Assessment
Lydia Clare Ellis Hill Farm Swinscoe Ashbourne Derbyshire DE6 2HH Geological Assessment: This report contains a geological description of the specified site or area. It is based on currently available 1:10 000 scale geological maps, unless otherwise stated, together with other relevant local information such as borehole records. The report includes extracts from digitised 1:50 000 scale geological maps (DigMapGB-50). The report contains the following modules: Geological Assessment (area) Geological Map Extracts Geoscience Data List Natural Ground Stability Report Id: GR_218809/1 Client reference: Search location This report describes a site located at National Grid Reference 413096, 345351. Note that for sites of irregular shape, this point may lie outside the site boundary. Where the client has submitted a site plan the assessment will be based on the area given. Search location indicated in red This product includes mapping data licensed from Ordnance Survey. © Crown Copyright and/or database right 2018. Licence number 100021290 EUL Scale: 1:5 000 (1cm = 50 m) Contains Ordnance Survey data © Crown Copyright and database right 2018 OS Street View: Scale: 1:5 000 (1cm = 50 m) Date: 24 July 2018 Page: 2 of 28 © NERC, 2018. All rights reserved. BGS Report No: GR_218809/1 Geological Map Extracts This part of the report contains extracts of geological maps taken from the 1:50 000 scale BGS Digital Geological Map of Great Britain (DiGMapGB-50). The geological information in DiGMapGB is separated into four themes: artificial ground, landslide deposits, superficial deposits and bedrock, shown here in separate maps. The fifth ‘combined geology’ map superimposes all four of these themes, to show the geological formations that occur at the surface, just beneath the soil. -

8. Hydrogeology and Land Contamination
Greenpark Energy Ltd April 2010 8. Hydrogeology and Land Contamination 8.1 Summary This chapter assessed the hydrogeological context of the proposed development together with possible impacts resulting from the development and considered the following key issues: The impact of the development on aquifers and groundwater located beneath the site; The impact of the development on nearby groundwater abstractions; Mitigation measures to be undertaken during site preparation, drilling and production and restoration of the site to prevent potential adverse impacts. This chapter also considered the current contamination status of the site and the potential for land contamination resulting from the proposed development and considers the following key issues: Potential contamination of land resulting from the proposed development; Mitigation measures to be undertaken during site preparation, drilling and production and restoration of the site to prevent potential adverse impacts. The site has variable shallow drift geology including glacial sand and gravel and glacial till to depths of between 0.9 and 2.6m below ground level. The solid geology below the site comprises strata of the Mercia Mudstone Group. The Mercia Mudstones overlie strata of the Triassic Sherwood Sandstone Group which comprises thick sandstones and is classified as a Major Aquifer by the Environment Agency; these strata extend up to approximately 300m in thickness in the area and are locally abstracted by Severn Trent Water for public potable supply. The Sherwood Sandstones lie unconformably above Upper Carboniferous Strata which include the Coal Measures Strata which are the target strata for the CBM gas development. The Coal Measures Strata are separated from the overlying Sherwood Sandstone by the Carboniferous Barren Measures Strata which are classified as a Minor Aquifer. -

Garforth and Church Fenton
High Speed Two Phase 2b ww.hs2.org.uk October 2018 Working Draft Environmental Statement High Speed Rail (Crewe to Manchester and West Midlands to Leeds) Working Draft Environmental Statement Volume 2: Community Area report | Volume 2 | LA16 LA16: Garforth and Church Fenton High Speed Two (HS2) Limited Two Snowhill, Snow Hill Queensway, Birmingham B4 6GA Freephone: 08081 434 434 Minicom: 08081 456 472 Email: [email protected] H27 hs2.org.uk October 2018 High Speed Rail (Crewe to Manchester and West Midlands to Leeds) Working Draft Environmental Statement Volume 2: Community Area report LA16: Garforth and Church Fenton H27 hs2.org.uk High Speed Two (HS2) Limited has been tasked by the Department for Transport (DfT) with managing the delivery of a new national high speed rail network. It is a non-departmental public body wholly owned by the DfT. High Speed Two (HS2) Limited, Two Snowhill Snow Hill Queensway Birmingham B4 6GA Telephone: 08081 434 434 General email enquiries: [email protected] Website: www.hs2.org.uk A report prepared for High Speed Two (HS2) Limited: High Speed Two (HS2) Limited has actively considered the needs of blind and partially sighted people in accessing this document. The text will be made available in full on the HS2 website. The text may be freely downloaded and translated by individuals or organisations for conversion into other accessible formats. If you have other needs in this regard please contact High Speed Two (HS2) Limited. © High Speed Two (HS2) Limited, 2018, except where otherwise stated. Copyright in the typographical arrangement rests with High Speed Two (HS2) Limited. -

1. Ripon, a City of Just Under 17,000 People, Is 54 Degrees N and 1.5 Degrees West and Sits Near to the Northern End of the Sout
RIPON CITY PLAN Supporting Document: Sustainable Ripon Appendix A.n: Ripon’s Geodiversity Introduction 1. Ripon, a city of just under 17,000 people, is 54 degrees N and 1.5 degrees west and sits near to the northern end of the Southern Magnesian Limestone National Character Area (NCA 30) defined by the underlying Permian Zechstein Group, formerly known as the Magnesian Limestone. It creates a very long and thin area stretching from Thornborough in North Yorkshire to the outskirts of Nottingham. The limestones, marls and enclosed gypsum horizons create a ridge, or narrow belt of elevated land, running north–south and forming a prominent landscape feature. The geology has influenced the form of the landscape, the use of limestone for local building stone and mortar, the specialised limestone grasslands, and in Ripon’s case, and the villages to the north and south, produces unstable land and a particular topography that has defined its settlement history and has geographically limited development. 2. The key to the unique landscape and development of the settlement of Ripon is its geodiversity, defined as earth materials, landforms and processes that shape the Earth. The geodiversity is manifested as the rocks, minerals, soils, topography, rivers and landscape of the area. Ripon sits at 30m above sea level on fluvio-glacial and river terraces that form the higher land between the Ure, Laver and Skell valleys. In the recent past these rivers cut deeply into the underlying Permian deposits, especially to the west of the City, when the flow greatly increased with meltwater from the glaciers of the last Ice Age only 13,000 years ago. -

Engineering Geological Aspects of Embankment Dams on Mercia Mudstone
Engineering Geological Aspects of Embankment Dams on Mercia Mudstone Tim Blower, CEng, MICE, CGeol, FGS Outline of Presentation • Introduction • Lincoln case study • Initial studies into Mercia Mudstone • Site investigations • Research on historical dams • Observations • Lincoln case study - reprise Embankment Dams on Mercia Mudstone Introduction • Lincoln WTW Project • Promoters are ANGLIAN WATER • Forecast supply deficit of 20 Ml/d by 2035 in Lincoln area • Business Case solution is a new 20 Ml/d treatment works in the Lincoln area • To satisfy future demand growth in this area, but also to improve resilience (increasing demand further south) Embankment Dams on Mercia Mudstone Newton Cliff SAM RWR Raw water pipeline Treated water pipeline New Pumping Station Existing WTW New river intake New WTW RWR Lincoln WTW Project – Storage Reservoir • Earth fill embankment dam • Roughly oval shape • 10 days storage including outage time • Capacity 285,000m 3 • Earthworks volumes - cut & fill: 220,000m 3 Embankment Dams on Mercia Mudstone Desk Study • Project Constraints • Risk Areas • Planning the GI • Initial studies on the Mercia Mudstone • Walkover survey Embankment Dams on Mercia Mudstone General References on Mercia Mudstone • Chandler, R.J. & Forster, A (2001). Engineering in Mercia mudstone. CIRIA Report C570. • Hobbs, P.R.N. et. al. (2001). Engineering Geology of British Rocks and Soils - Mudstones of the Mercia Mudstone Group; BGS Research Report RR/01/02. • Howard, A.S. et.al. (2008). A Formational Framework for the Mercia Mudstone Group (Triassic) of England and Wales; BGS Research Report RR/08/04. Embankment Dams on Mercia Mudstone Outcrop of Mercia Mudstone 10 MM age from Mid Triassic (Anisian) to latest Triassic 9 (Rhaetian) 8 6 (241 – 205 mybp). -

Western Link
Western Link Ground Investigation Report 20 October 2017 Warrington Borough Council Mott MacDonald Spring Bank House 33 Stamford Street Altrincham WA14 1ES United Kingdom T +44 (0)161 926 4000 F +44 (0)161 929 8915 mottmac.com Warrington Borough Council Warrington 26-30 Horsemarket Street Western Link WA1 1XL 382900-WL-MMD-07-ZZ-RP-N-0002 https://mottmac.sharepoint.com/teams/pj-b1092/Shared Documents/12 - Geotechnics/Reports/Documents/382900-WL-MMD-07-ZZ-RP-N-0002 GIR.docx Mott MacDonald Ground Investigation Report 20 October 2017 Mott MacDonald Limited. Registered in England and Wales no. 1243967. Registered office: Mott MacDonald House, 8-10 Sydenham Road, Croydon CR0 2EE, Warrington Borough Council United Kingdom Mott MacDonald | Western Link Ground Investigation Report Contents Executive summary 1 1 Introduction 3 1.1 Scope and Objective of the Report 3 1.2 Description of Project 3 1.3 Geotechnical Category of Project 4 2 Existing Information 5 2.1 Topographic Maps (Old and Recent) 5 2.2 Geological Maps and Memoirs 5 2.3 Hydrology 6 2.4 Hydrogeology 6 2.5 Records of Mines and Mineral Deposits 6 2.6 Land Use and Soil Survey Information 7 2.7 Archaeological and Historical Investigations 7 2.8 Existing Ground Investigations 8 2.9 Consultation with Statutory Bodies and Agencies 8 2.10 Flood Records 8 2.11 Contaminated Land 8 3 Field and Laboratory Studies 9 3.1 Walkover Survey 9 3.2 Ground Investigation 9 3.3 Other Field Work 10 3.4 Laboratory Investigation 10 4 Ground Summary 12 4.1 Overview 12 5 Ground Conditions and Material Properties -
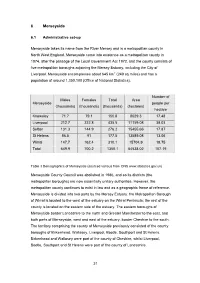
6 Merseyside
6 Merseyside 6.1 Administrative set-up Merseyside takes its name from the River Mersey and is a metropolitan county in North West England. Merseyside came into existence as a metropolitan county in 1974, after the passage of the Local Government Act 1972, and the county consists of five metropolitan boroughs adjoining the Mersey Estuary, including the City of Liverpool. Merseyside encompasses about 645 km2 (249 sq miles) and has a population of around 1,350,100 (Office of National Statistics). Number of Males Females Total Area Merseyside people per (thousands) (thousands) (thousands) (hectares) hectare Knowsley 71.7 79.1 150.8 8629.3 17.48 Liverpool 212.7 222.8 435.5 11159.08 39.03 Sefton 131.3 144.9 276.2 15455.66 17.87 St Helens 86.5 91 177.5 13589.08 13.06 Wirral 147.7 162.4 310.1 15704.9 19.75 Total 649.9 700.2 1350.1 64538.02 107.19 Table 3 Demographics of Merseyside (sourced various from ONS www.statistics.gov.uk) Merseyside County Council was abolished in 1986, and so its districts (the metropolitan boroughs) are now essentially unitary authorities. However, the metropolitan county continues to exist in law and as a geographic frame of reference. Merseyside is divided into two parts by the Mersey Estuary: the Metropolitan Borough of Wirral is located to the west of the estuary on the Wirral Peninsula; the rest of the county is located on the eastern side of the estuary. The eastern boroughs of Merseyside border Lancashire to the north and Greater Manchester to the east, and both parts of Merseyside, west and east of the estuary, border Cheshire to the south. -

Using Core and Outcrop Analogues to Predict Flow Pathways in the Subsurface: Examples from the Triassic Sandstones of North Ches
Adv. Geosci., 49, 121–127, 2019 https://doi.org/10.5194/adgeo-49-121-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. Using core and outcrop analogues to predict flow pathways in the subsurface: examples from the Triassic sandstones of north Cheshire, UK Joanna Thompson, Daniel Parkes, Edward Hough, and Oliver Wakefield British Geological Survey, Keyworth, Nottingham, NG12 5GG, UK Correspondence: Joanna Thompson ([email protected]) Received: 30 May 2019 – Accepted: 2 August 2019 – Published: 19 September 2019 Abstract. Borehole core provides detailed vertical data resources such as pore space and heat extraction. Subsurface which is used to interpret subsurface sand body architec- fluid flow is relevant to the development of new, low-carbon tures, but assumptions are made on the relationship between energy technologies (e.g., geothermal systems located in sed- the lateral and vertical thickness, and the interconnectivity imentary aquifers, underground gas storage and Carbon Cap- of units. The sedimentological complexity of the Sherwood ture and Storage; CCS). In the UK, there is the potential for Sandstone Group succession in this area, passing between the development of these geological settings where there are aeolian and fluvial packages creates local- to regional-scale porous and permeable rocks that may serve as production or heterogeneities which will impact flow pathways within the storage horizons located in proximity to sources of energy rockmass. Measured thickness in boreholes might represent such as shale gas reservoirs, coal seams or renewables; op- an architectural element’s true maximum thickness or more erational viability is increased where these geologies are lo- likely, a partial thickness as a result of incision by over- cated near areas of industrial activity or areas of dense popu- lying facies types or as a result of the borehole sitting to- lation. -
IAEG2006 Field Trip Guide Ripon 1 GYPSUM DISSOLUTION
CORE Metadata, citation and similar papers at core.ac.uk Provided by NERC Open Research Archive IAEG2006 Field Trip Guide Ripon GYPSUM DISSOLUTION GEOHAZARDS AT RIPON, NORTH YORKSHIRE, UK Anthony H.Cooper1 1British Geological Survey, Keyworth, Nottingham, NG12 5GG, e-mail:[email protected] Abstract: This guide is for a one-day field excursion to examine gypsum dissolution geohazards at Ripon in North Yorkshire. Gypsum is a highly soluble rock and under suitable groundwater flow conditions it can dissolve forming caves and karstic features including collapse and suffosion dolines. These have the capability of causing subsidence damage of the type that affects much of the Ripon area. The guide details the processes involved, the localities visited and some of the remedial measures undertaken. Résumé: Ce guide concerne l'excursion d'une journée ayant pour but d'étudier les géo-aléas liés à la dissolution du gypse à Ripon dans la région du North Yorkshire. Le gypse est une roche hautement soluble et, dans des conditions adéquates d'écoulement de l'eau souterraine, pouvant se dissoudre et engendrer des grottes et formes karstiques telles qu'effondrements et dolines de suffosion. Celles-ci peuvent causer des dégâts de subsidence du type de ceux qui touchent la plupart des alentours de Ripon. Ce guide fournit des explications sur les phénomènes mis en jeu, les localités visitées et quelques-unes des mesures prises pour remédier à ces problèmes. Keywords: subsidence, evaporites, aquifers, cavities, collapse, urban geosciences. INTRODUCTION Gypsum, hydrated Calcium Sulphate (CaSO4.2H2O), is attractive as satin spar, beautiful as carved alabaster, practical as plasterboard (wallboard), but the cause of a geological hazard capable of swallowing houses and collapsing dams.