Federal Register/Vol. 84, No. 78/Tuesday, April 23, 2019/Rules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
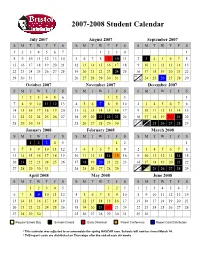
Approved Student Calendar
2007-2008 Student Calendar July 2007 August 2007 September 2007 SMTWT F S SMTWT F S SMTWT F S 1234567 1234 1 8910111213145678910 11 2 3 45678 15 16 17 18 19 20 21 12 13 14 15 16 17 18 9 10 11 12 13 14 15 22 23 24 25 26 27 28 19 20 21 22 23 24 25 16 17 18 19 20 21 22 23 29 30 31 26 27 28 29 30 31 30 24 25 26 27 28 29 October 2007 November 2007 December 2007 SMTWT F S SMTWT F S SMTWT F S 123456 123 1 7891011 12 134567 89102345678 14 15 16 17 18 19 20 11 12 13 14 15 16 17 9 10 11 12 13 14 15 21 22 23 24 25 26 27 18 19 20 21 22 23 24 16 17 18 19 20 21 22 23 24 28 29 30 31 25 26 27 28 29 30 30 31 25 26 27 28 29 January 2008 February 2008 March 2008 SMTWT F S SMTWT F S SMTWT F S 12345 12 1 67891011123456789 2345678 13 14 15 16 17 18 19 10 11 12 13 14 1516 9 1011121314 15 20 21 22 23 24 25 26 17 18 19 20 21 22 23 16 17 18 19 20 21 22 23 24 27 28 29 30 31 24 25 26 27 28 29 30 31 25 26 27 28 29 April 2008 May 2008 June 2008 SMTWT F S SMTWT F S SMTWT F S 12345 123 1234567 6789 10111245678910891011121314 13 14 15 16 17 18 19 11 12 13 14 15 16 17 15 16 17 18 19 20 21 20 21 22 23 24 25 26 18 19 20 21 22 23 24 22 23 24 25 26 27 28 27 28 29 30 25 26 27 28 29 30 31 29 30 Regular School Day Schools Closed Early Dismissal Parent Conference Report Card Distribution * This calendar was adjusted to accommodate the spring NASCAR race. -

Federal Register/Vol. 86, No. 82/Friday, April 30, 2021/Rules And
22866 Federal Register / Vol. 86, No. 82 / Friday, April 30, 2021 / Rules and Regulations submission must indicate whether the Secretariat Chemin de Blandonnet 8 CP Room 820, 4330 East West Highway, rule is a ‘‘major rule.’’ The CRA states 401—1214 Vernier, Geneva, Bethesda, MD 20814, telephone (301) that the Office of Information and Switzerland; Telephone + 41 22 749 01 504–7479, email: [email protected], or Regulatory Affairs (OIRA) determines 11, Fax + 41 22 733 34 30; http:// at the National Archives and Records whether a rule qualifies as a ‘‘major www.iso.org/iso/home.htm. Administration (NARA). For rule.’’ (i) ISO/IEC 17011:2017(E) (ISO/IEC information on the availability of this Pursuant to the CRA, this rule does 17011), ‘‘Conformity assessment— material at NARA, email fedreg.legal@ not qualify as a ‘‘major rule,’’ as defined Requirements for accreditation bodies nara.gov, or go to: www.archives.gov/ in 5 U.S.C. 804(2). To comply with the accrediting conformity assessment federal-register/cfr/ibr-locations.html. CRA, CPSC will submit the required bodies,’’ November 10, 2017; and information to each House of Congress (ii) ISO/IEC 17025:2017(E) (ISO/IEC § 1112.43 [Amended] and the Comptroller General. 17025), ‘‘General requirements for the ■ 7. In § 1112.43(a)(3), remove the List of Subjects competence of testing and calibration phrase ‘‘ISO/IEC 17025:2005(E)’’ and laboratories,’’ November 10, 2017. add in its place the phrase ‘‘ISO/IEC 16 CFR Part 1107 (2) [Reserved] 17025:2017(E)’’. Business and industry, Children, § 1107.26 [Amended] Alberta E. -

Planning for the 2021-2022 School Year Florida Continues To
State Board of Education Richard Corcoran Commissioner of Education Andy Tuck, Chair Marva Johnson, Vice Chair Members Monesia Brown Ben Gibson Tom Grady Ryan Petty Joe York MEMORANDUM TO: FROM: Richard Corcoran DATE: April 14, 2021 SUBJECT: Planning for the 2021-2022 School Year Florida continues to outwork and outperform the nation in the number of students receiving a high-quality education in an in-person educational setting, and our success has been rooted in schools, districts and the state implementing learned best practices and constantly relying on science and evidence. Throughout the successful reopening ofour schools for in-person instruction, we have consistently provided families with the ability to make educational decisions that are in the best interest oftheir children. Our efforts ensured parents had the ability to choose from multiple learning modality options for the current school year, with the option to transition to new modalities when their child may have required another option to ensure they were achieving adequate progress. Florida has once again proven that one-size-fits-all policies do not meet the unique needs ofindividual students or their families. Therefore, we should continue to make surgical - not sweeping - decisions to mitigate large-scale educational disruptions as we are planning for the upcoming 2021-2022 school year. As you reflect on the current school year and look ahead to 2021-2022, an example ofa one-size-fits-all policy are the mandatory face covering policies in some districts and schools. Upon reviewing the policies of those districts with mandatory face covering policies, reviewing all districts relevant health data, and factoring in such data points as the percentage ofstudents learning in-person and the relative population of a county (which is often synonymous with a county's community health resources), the data shows us that districts' face covering policies do not impact the spread of the virus. -

April 14, 2021
April 14, 2021 As of yesterday's report, Greenwood County had 13 additional confirmed cases in the county for a total of 7,217 cases confirmed since 21 March 2020. Remember this is only those which have been confirmed and is for those who were tested a minimum of two days ago. This means there are others who have been tested and are in quarantine awaiting results. Additionally, an individual can be asymptomatic, (contagious but shows no symptoms), and may choose not to be tested. The first 7,070 cases in Greenwood County were reported over 15 days ago, so there are 7,070 individuals who should have recovered from the virus. Additionally, Greenwood County has had 150 confirmed deaths due to the virus. This brings Greenwood County's remaining estimated total of active confirmed cases to 146. DHEC does not track recovered individuals by county, so this number is an estimate based on the following: Most individuals recover from the virus within this time frame. From 28 March until 10 April (2 weeks), Greenwood has had 139 confirmed cases out of a population of 70,811. This equates to an incident rate of 196.3 per 100,000 individuals which is rated as medium by SCDHEC. (Low = 50 or less; Medium = 51 - 200; High = 201 or more) SCDHEC reported an additional 447 new confirmed cases in the state for a total of 472,310. Also, they reported 12 new confirmed deaths in the state for a total of 8,177. Please click this link to see the deaths by county. -
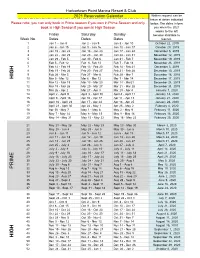
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
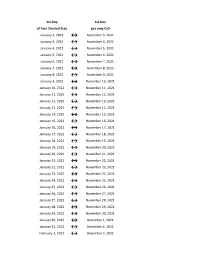
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 2, 2022 ↔ November 3, 2021 January 3, 2022 ↔ November 4, 2021 January 4, 2022 ↔ November 5, 2021 January 5, 2022 ↔ November 6, 2021 January 6, 2022 ↔ November 7, 2021 January 7, 2022 ↔ November 8, 2021 January 8, 2022 ↔ November 9, 2021 January 9, 2022 ↔ November 10, 2021 January 10, 2022 ↔ November 11, 2021 January 11, 2022 ↔ November 12, 2021 January 12, 2022 ↔ November 13, 2021 January 13, 2022 ↔ November 14, 2021 January 14, 2022 ↔ November 15, 2021 January 15, 2022 ↔ November 16, 2021 January 16, 2022 ↔ November 17, 2021 January 17, 2022 ↔ November 18, 2021 January 18, 2022 ↔ November 19, 2021 January 19, 2022 ↔ November 20, 2021 January 20, 2022 ↔ November 21, 2021 January 21, 2022 ↔ November 22, 2021 January 22, 2022 ↔ November 23, 2021 January 23, 2022 ↔ November 24, 2021 January 24, 2022 ↔ November 25, 2021 January 25, 2022 ↔ November 26, 2021 January 26, 2022 ↔ November 27, 2021 January 27, 2022 ↔ November 28, 2021 January 28, 2022 ↔ November 29, 2021 January 29, 2022 ↔ November 30, 2021 January 30, 2022 ↔ December 1, 2021 January 31, 2022 ↔ December 2, 2021 February 1, 2022 ↔ December 3, 2021 1st Day 1st Day of Your Desired Stay you may Call February 2, 2022 ↔ December 4, 2021 February 3, 2022 ↔ December 5, 2021 February 4, 2022 ↔ December 6, 2021 February 5, 2022 ↔ December 7, 2021 February 6, 2022 ↔ December 8, 2021 February 7, 2022 ↔ December 9, 2021 February 8, 2022 ↔ December 10, 2021 February 9, 2022 ↔ December 11, 2021 February 10, 2022 ↔ December 12, 2021 February -

Christian Brothers Academy 2020-2021 Calendar (April – June)
Christian Brothers Academy 2020-2021 Calendar (April – June) April 2021 Mon 5 Easter Monday - No School Tues 6 Return to Everyday Instruction Admissions Letters of Regular Decision Mailed Junior High Football and Junior High Volleyball Begin Fri 9 Junior High Event Wed 14 9th Grade Retreat Mon 19 High School Spring Sports Begin Wed 21 8th Grade NYS ELA Exam Thurs 22 High School Honors Ceremony Fri 23 Staff Development Day – No School Sat 24 9th Grade Event Virtual Lasallian Auction Begins Sun 25 Chorus/Orchestra/Band/Jazz Band Concert Thurs 29 Senior Retreat Fri 30 Registration for Junior High Spring Sports Ends Alumni Senior Luncheon May 2021 Sat 1 Virtual Lasallian Auction Program/Conclusion Mon 3 Board of Trustees Meeting 4PM Junior High Spring Sports Begin Mon-Fri 3-17/25 AP Exams Fri 7 First Friday Mass 7:30AM Sat 8 10th Grade Event Wed 12 8th Grade NYS Math Exam Thurs 13 Ascension Thursday Mass 9:30AM Fri 14 Junior High Event – Talent Show Junior Prom Mon 17 Founder’s Day Seniors’ Last Day of Classes Wed-Fri 19-21 Senior Exams Thurs 20 Art Expose Fri 21 Senior Ball Sat 22 Spring Musical Tues 25 Spring Cabaret Fri 28 Staff Development Day – No School Mon 31 Memorial Day – No School June 2021 Tues/Wed 1/2 8th Grade Moving Up Ceremony Wed 2 Songwriters’ Showcase Fri 4 Junior High Ice Cream Social First Friday Mass 7:30AM Senior Breakfast and Graduation Rehearsal Sat 5 Baccalaureate Mass 7PM Sun 6 Graduation Mon 7 Incoming 7th Grade Student/Parent Orientation Wed 9 Last Day of Classes Thurs 10 Study Day – No School Fri-Tues 11-24 School Final Exams & Regents Exams Fri 25 Grading Day July 2021 Thurs 1 Counselors’ Last Day *All events are subject to change based upon public health and safety guidelines. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

April 23, 1955 Zhou Enlai's Speech at The
Digital Archive digitalarchive.wilsoncenter.org International History Declassified April 23, 1955 Zhou Enlai’s Speech at the Political Committee of the Afro- Asian Conference Citation: “Zhou Enlai’s Speech at the Political Committee of the Afro-Asian Conference,” April 23, 1955, History and Public Policy Program Digital Archive, PRC FMA 207-00006-04, 69-75. Translated by Jeffrey Wang. http://digitalarchive.wilsoncenter.org/document/114678 Summary: Zhou Enlai discussed communist expansion, subversive activities and the prospect of peace, during which he mentioned the relation between Pakistan and China. He also put forth the Chinese motion that the Conference should have a peace declaration of seven points: Mutual respect of sovereignty and territorial integrity, mutual non-adoption of invasive action or threats, mutual non-interference of internal affairs, acknowledgment of racial equality, acknowledgment of equality of countries, recognition of people's right to decide their own political and economic systems, and mutual non- detriment. Credits: This document was made possible with support from the MacArthur Foundation. Original Language: Chinese Contents: English Translation [...] Chairman, Delegates, Yesterday and today I heard opinions from many representatives. I have also read the motion brought forth by eight delegations. I am now willing to combine everyone’s common opinions and bring forth the motion from the Chinese delegation. The current world situation is indeed tense. However, peace is not without hope, and those who protect peace are increasing in numbers. 29 Afro-Asian countries are here together in a meeting, all calling for peace. This proves that over half of the world’s population wants peace and solidarity.