Are Macrophyte-Dwelling Chironomidae (Diptera) Largely Opportunistic in Selecting Plant Species?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diptera: Corethrellidae) Author(S): Priyanka De Silva and Ximena E
First Report of the Mating Behavior of a Species of Frog-Biting Midge (Diptera: Corethrellidae) Author(s): Priyanka De Silva and Ximena E. Bernal Source: Florida Entomologist, 96(4):1522-1529. 2013. Published By: Florida Entomological Society DOI: http://dx.doi.org/10.1653/024.096.0434 URL: http://www.bioone.org/doi/full/10.1653/024.096.0434 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. 1522 Florida Entomologist 96(4) December 2013 FIRST REPORT OF THE MATING BEHAVIOR OF A SPECIES OF FROG-BITING MIDGE (DIPTERA: CORETHRELLIDAE) PRIYANKA DE SILVA1,* AND XIMENA E. BERNAL1, 2 1Department of Biological Science, Texas Tech University, P.O. Box 43131, Lubbock, TX, 79409, USA 2Smithsonian Tropical Research Institute, Apartado 2072, Balboa, Republic of Panama *Corresponding author; E-mail: [email protected] ABSTRACT Swarming is a common mating behavior present throughout Diptera and, in particular, in species of lower flies (Nematocerous Diptera). -

Biological Monitoring of Surface Waters in New York State, 2019
NYSDEC SOP #208-19 Title: Stream Biomonitoring Rev: 1.2 Date: 03/29/19 Page 1 of 188 New York State Department of Environmental Conservation Division of Water Standard Operating Procedure: Biological Monitoring of Surface Waters in New York State March 2019 Note: Division of Water (DOW) SOP revisions from year 2016 forward will only capture the current year parties involved with drafting/revising/approving the SOP on the cover page. The dated signatures of those parties will be captured here as well. The historical log of all SOP updates and revisions (past & present) will immediately follow the cover page. NYSDEC SOP 208-19 Stream Biomonitoring Rev. 1.2 Date: 03/29/2019 Page 3 of 188 SOP #208 Update Log 1 Prepared/ Revision Revised by Approved by Number Date Summary of Changes DOW Staff Rose Ann Garry 7/25/2007 Alexander J. Smith Rose Ann Garry 11/25/2009 Alexander J. Smith Jason Fagel 1.0 3/29/2012 Alexander J. Smith Jason Fagel 2.0 4/18/2014 • Definition of a reference site clarified (Sect. 8.2.3) • WAVE results added as a factor Alexander J. Smith Jason Fagel 3.0 4/1/2016 in site selection (Sect. 8.2.2 & 8.2.6) • HMA details added (Sect. 8.10) • Nonsubstantive changes 2 • Disinfection procedures (Sect. 8) • Headwater (Sect. 9.4.1 & 10.2.7) assessment methods added • Benthic multiplate method added (Sect, 9.4.3) Brian Duffy Rose Ann Garry 1.0 5/01/2018 • Lake (Sect. 9.4.5 & Sect. 10.) assessment methods added • Detail on biological impairment sampling (Sect. -

DNA Barcoding
Full-time PhD studies of Ecology and Environmental Protection Piotr Gadawski Species diversity and origin of non-biting midges (Chironomidae) from a geologically young lake PhD Thesis and its old spring system Performed in Department of Invertebrate Zoology and Hydrobiology in Institute of Ecology and Environmental Protection Różnorodność gatunkowa i pochodzenie fauny Supervisor: ochotkowatych (Chironomidae) z geologicznie Prof. dr hab. Michał Grabowski młodego jeziora i starego systemu źródlisk Auxiliary supervisor: Dr. Matteo Montagna, Assoc. Prof. Łódź, 2020 Łódź, 2020 Table of contents Acknowledgements ..........................................................................................................3 Summary ...........................................................................................................................4 General introduction .........................................................................................................6 Skadar Lake ...................................................................................................................7 Chironomidae ..............................................................................................................10 Species concept and integrative taxonomy .................................................................12 DNA barcoding ...........................................................................................................14 Chapter I. First insight into the diversity and ecology of non-biting midges (Diptera: Chironomidae) -

Chironomidae: Diptera)
Ann. Limnol. - Int. J. Lim. 46 (2010) 181–190 Available online at: Ó EDP Sciences, 2010 www.limnology-journal.org DOI: 10.1051/limn/2010015 Colonisation of temporary macrophyte substratum by midges (Chironomidae: Diptera) Dubravka Cˇ erba1, Zlatko Mihaljevic´ 2* and Jasna Vidakovic´ 1 1 Department of Biology, Josip Juraj Strossmayer University, Trg Ljudevita Gaja 6, HR-31000 Osijek, Croatia 2 Department of Zoology, Faculty of Science, University of Zagreb, Rooseveltov trg 6, HR-10000 Zagreb, Croatia Received 2 February 2010; Accepted 18 May 2010 Abstract – This study investigates a phytophylous community of Chironomidae larvae on the submerged plant species Myriophyllum spicatum L. in a eutrophic lake (Lake Sakadasˇ , Danube floodplain area in Croatia) during summer 2004. This macrophyte species appeared for the first time in the lake in 2004, lasted approximately three months and was considered as a temporary habitat. The chironomid community was very abundant in the stands of this macrophyte species, which developed at three sites. The recorded species belong to three subfamilies: Chironominae (Chironomini and Tanytarsini), Orthocladiinae and Tanypodinae. Species composition varied in time, though Orthocladiinae with their representative Cricotopus sylvestris gr. dominated throughout the entire sampling period, accounting for approx. 60% of the total community. However, the presence of this species group experienced a marked drop to only 10% in September when Chironomini larvulae and Paratanytarsus sp. prevailed. Furthermore, the share of Endochironomus albipennis (Meigen 1830) in relation to other species was also consistently higher. NMDS ordination and cluster analysis separated three main colonization periods based on larval abundance. RDA analysis indicated the influence of environmental variables, especially Secchi depth, macrophyte dry weight, depth and water temperature, on chironomid community structure. -

Chironomus Frontpage No 28
CHIRONOMUS Journal of Chironomidae Research No. 28 ISSN 0172-1941 (printed) 2387-5372 (online) December 2015 CONTENTS Editorial Online early - only online 3 Current Research Krosch, M.N. & Bryant, L.M. Note on sampling chironomids for RNA-based studies of natural populations that retains critical morphological vouchers 4 da Silva, F.L. et al. A preliminary survey of the non-biting midges of the Dominican Republic 12 da Silva, F.L. et al. Chironomidae types in the reference collection of the laboratory of ecology of aquatic insects, São Carlos, Brazil 20 Short Communications de la Rosa, C.L. Chironomids: a personal journey 30 Kondrateva, T.A. & Nazarova, L.B. Preliminary data on the chironomid fauna of the Middle Volga region within the Republic of Tatarstan (Russia) 36 Martin, J. Identification of Chironomus (Chironomus) melanescens Keyl, 1962 in North America 40 Syrovátka, V. & Langton, P.H. First records of Lasiodiamesa gracilis, Parochlus kiefferi and several other Chironomidae from the Czech Republic and Slovakia 45 Murray, D.A. Lost and found in Ireland; how a data label resulted in a postal delivery to Metriocnemus (Inermipupa) carmencitabertarum 57 In memoriam 60 Stenochironomus sp. from La Selva Research Station, Costa Rica. Photo: Carlos de la Rosa. CHIRONOMUS Journal of Chironomidae Research Editors Alyssa M. ANDERSON, Department of Biology, Chemistry, Physics, and Mathematics, Northern State University, Aberdeen, South Dakota, USA. Torbjørn EKREM, NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway. Peter H. LANGTON, 16, Irish Society Court, Coleraine, Co. Londonderry, Northern Ireland BT52 1GX. The CHIRONOMUS Journal of Chironomidae Research is devoted to all aspects of chironomid research and serves as an up-to-date research journal and news bulletin for the Chironomidae research community. -

Appendix a – Field Survey Chronology
APPENDIX A – FIELD SURVEY CHRONOLOGY Cambridge West MESP November 2013 Natural Environment Veg / Flora Avifauna Herpetofauna Mammals Aquatics Salamander Road Mortality Date Staff Task Owl MMP Badger Benthics Nocturnal Reptile Cover BoardCover Breeding Breeding Bird Fall Fall Spawning Supplemental (incl. (incl. migrant) Turtle Nesting Turtle Basking Amphibian ELC / ELC Botanical Assessment Trap Setting Aquatic Habitat General Wildlife General Lepidoptera / Odonata Amphibian Calling Pitfall TrappingPitfall Pond Trapping / Feature Delineation 2008 Apr-22 KLF/RH Site reconnaissance and Calling Amphibian Survey # 1 – 9 sites 6 Apr-23 KLF Vernal pond reconnaissance assessment 1 May-29 HA Breeding Bird Survey #1 6 Jun-04 KLF/EW Calling Amphibian Survey # 2 6 Vegetation Survey - ELC mapping, spring vegetation inventory Jun-12 RH, LV 8 7 Breeding Bird Survey # 2 Jun-23 KLF/EW Calling Amphibian Survey # 3; Road / mortality survey. 5 2 Jul-24 KLF/SD Dipnetting vernal ponds 5.5 Site Reconnaissance - aquatic habitat / survey locations; vernal pond Aug-12 CC/KLE 4 7 habitat assessment / survey locations Vegetation (ELC) and botanical inventory – central pond; general wildlife Sep-19 JG 2 0.5 survey 2009 Mar-10 KLF/KH/KD Site Reconnaissance & pond (minnow) trap setting. 15 3 Mar-11 KLF/EG Pond (minnow) trap survey / check and closing 11 Mar-18 KLF/EG/ Pond (minnow) trap setting 15 ECOPLANS Table A-1. Natural Environment Field Survey Chronology Page A -1 Cambridge West MESP November 2013 Natural Environment Veg / Flora Avifauna Herpetofauna Mammals Aquatics Salamander Road Mortality Date Staff Task Owl MMP Badger Benthics Nocturnal Reptile Cover BoardCover Breeding Breeding Bird Fall Fall Spawning Supplemental (incl. -

Phenology of Non-Biting Midges (Diptera: Chironomidae) in Peatland Ponds, Central Poland
© Entomologica Fennica. 1 June 2018 Phenology of non-biting midges (Diptera: Chironomidae) in peatland ponds, Central Poland Mateusz P³óciennik, Martyna Skonieczka, Olga Antczak & Jacek Siciñski P³óciennik, M., Skonieczka, M., Antczak, O. & Siciñski, J. 2018: Phenology of non-biting midges (Diptera: Chironomidae) in peatland ponds, Central Poland. Entomol. Fennica 29: 6174. Non-biting midges are one ofthe most diverse and abundant aquatic insects in peatlands. The R¹bieñ mire is a raised bog located on the edge ofthe Lodz Ag - glomeration in Central Poland. After peat extraction, many ponds remained in the R¹bieñ area. During the growing season in 2012, adult chironomids were col- lected by a light trap and a hand net near one ofthe excavation ponds. The pheno - logy of adult flight period was documented from April to November. Thirty-one species were recorded and assigned to one offivephenology groups. Three pa- rameters reflecting duration of daytime and weather conditions, i.e. air tempera- ture, air humidity, were found to covary significantly with the observed flight pe- riods. Taxa emerging in the spring may be classified as cold-adapted and those collected in the summer only as preferring higher air temperature. Emergence in late summer was related to a shorter duration ofdaytime. M. P³óciennik, O. Antczak & J. Siciñski, Department of Invertebrate Zoology and Hydrobiology, University of Lodz, 12/16 Banacha St., Lodz 90-237, Poland; E- mails: [email protected], [email protected], sicinski@bio- l.uni.lodz.pl M. Skonieczka, 22/61 Pi³sudskiego St., Aleksandrów £ódzki, 95-070, Poland; e- mail: [email protected] Received 21 December 2016, accepted 27 September 2017 1. -
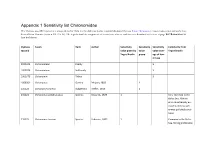
Appendix 1 Sensitivity List Chironomidae the Dyntaxa Taxonid Represents a Unique Identifier from the Swedish Taxonomic Standard Database Dyntaxa (
Appendix 1 Sensitivity list Chironomidae The Dyntaxa taxonID represents a unique identifier from the Swedish taxonomic standard database Dyntaxa (http://dyntaxa.se). Taxon names rank and author are derived from Dyntaxa (version 2013-06-26). The logic behind the assignment of a sensitivity class to each taxon is described in the text at page Fel! Bokmärket är inte definierat.. Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa 2001302 Chironomidae Family 5 1009974 Chironominae Subfamily 5 1009975 Chironomini Tribus 5 1009301 Chironomus Genera Meigen, 1803 1 235223 Camptochironomus Subgenera Kieffer, 1918 1 235224 Chironomus pallidivittatus Species Edwards, 1929 1 Very common in the Baltic Sea. Able to endure extremely eu- trophic and also oth- erwise polluted condi- tions. 235225 Chironomus tentans Species Fabricius, 1805 1 Common in the Baltic Sea. Strong preference WATERS: A PROBABILITY BASED INDEX FOR BENTHIC ASSESSMENT IN THE BALTIC SEA Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa for eutrophic and even extremely eutrophic and polluted condi- tions. 235228 Chironomus Subgenera Meigen, 1803 1 235234 Chironomus annularius Species Meigen, 1818 1 Rather common in the Baltic Sea, easily con- fused with several other Chironomus species bout as larvae and adults. 235235 Chironomus anthracinus Species Zetterstedt, 1860 1 Common north of Åland, otherwise less common in the Baltic Sea. Mainly found below the littoral. Prefers less strongly 2 WATERS: A PROBABILITY BASED INDEX FOR BENTHIC ASSESSMENT IN THE BALTIC SEA Dyntaxa Taxon Rank Author Sensitivity Sensitivity Sensitivity Comments from taxonid value given by value value aver- Yngve Brodin Yngve Brodin group age of low- er taxa eutrophic conditions than C. -

FEEDING BEHAVIOUR OF'breeding WIGEON Anas Penelope in RELATION to SEASONAL EMERGENCE and SWARMING BEHAVIOUR of CHIRONOMIDS
409 FEEDING BEHAVIOUR OF'BREEDING WIGEON Anas penelope IN RELATION TO SEASONAL EMERGENCE AND SWARMING BEHAVIOUR OF CHIRONOMIDS ODD W. JACOBSEN ABSTRACT Emergence ofchironomids started in mid-April, and reached a peak at the end of April and in the beginning of May. Most "spring" chiro nomids emerged around noon, whereas the majority of chironomids emerg ing later in the season, appeared at night. In general, there was a positive correlation between the seasonal emergencl~ of chironomids and gleaning frequency by individual Wigeon. Gleaning was observed exclusively on emergent vegetation in the morning and evening. During midday, gleaning occurred most frequently on water surface probably due to the diel emergence and swarming patterns ofchironomids. Gleaning was most frequently used by females, which is mainly due to their higher energetic requirements during the breeding seasons. I conclude that the increasing abundance of emerging chironomids in the spring is one important factor affecting time of nesting by Wigeon. Dept. of Animal Ecology, Museum of Zoology, University of Bergen, N 5007 Bergen, Norway. INTRODUCTION several weeks and months before migration to the Arctic (Raveling 1979, Ankney 1984). Most dab Patterns in avian reproduction ultimately are adapt bling ducks, however, obtain their requirements ed to and proximately generated by variations in (lipids, proteins, minerals) for breeding by storing food supply (Lack 1966, Daan et al. 1988). In order reserves and by utilizing resources on their breed to understand avian breeding strategies, it is impor ing grounds (Drobney 1980, Krapu 1981). Although tant to know how resources are distributed in space recent studies have shown that food quality in the and time and how the birds obtain them during their non-breeding season may influence timing and re breeding cycle (Alisauskas & Ankney 1985). -

Mitteilungen Aus Der Chironomidenkunde Newsletter of Chironomid Research
MITTEILUNGEN AUS DER CHIRONOMIDENKUNDE NEWSLETTER OF CHIRONOMID RESEARCH CHIRONOMUS Vol. 2 No.3 Miinchen, Marz 1982 CHIP-ONOMID RESEARCH 11 THE NETHERLANDS Bernard P.M. Krebs Chironomid research in the Netherlands goes back to Van der Wulp, who wrote various dipterological papers, amongst others several about chironomids. In 1898 he and De Mei,jere published a checklist of Dutch diptera's. Irl rece11L chir.onomid literature we can find his name as the author of different Orthocladiinae genera, e.g. Cri~otopus and Orthocladius and difrerenl species like Peiltapedilum sordens and Parachironomus -7 monochromus. In 1928 De MeiJere jnsist.ed in a paper on a revision of the Dutch Tendipedidae. Kruseman took up this gauntlet and in l933 he published his study about the "Tendipedirlae Neerlandicae". Quite different was the research of Van der Torren in the same period. He studied the midge-plagues caused by the closure and desalinisation of the Zuyderzee (now Lake Yssel). -n his unpublished report he de- scribed and discussed the symptoms, the abatement and the possibility of a second outbreak of this plague. At the end of the sixties new research was set up. The recent research can be dis- tinguished roughly in three classes: A) Applied systematical research for water quality and nature management, B) Fundamental research like chironomid ecology and taxonomy, C) Fundamental ecosystem research, of which the chironomid st,~dyis part of the whole research. A) Due to the large numbers of species (in the Ketherlands more than 400) and the possibilities to use the species for characterization of water quality and water economy, many research workers of (mostly) local governments, who are responsible for water q,~ality,are making a study of the distribution and ecclogy of chirono- mid larvae (and other groups of the macrofauna) In all parts of the country. -

MOLECULAR IDENTIFICATION of CHIRONOMID SPECIES BASED on ITS-1 and ITS-2 REGIONS of Rdna
MOLECULAR IDENTIFICATION OF CHIRONOMID SPECIES BASED ON ITS-1 AND ITS-2 REGIONS OF rDNA A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By MONITA SHARMA B.Sc. Maharani College, Jaipur, India 2001 2007 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES May 15, 2007 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Monita Sharma ENTITLED Molecular Identification of Chironomid species based on ITS-1 and ITS-2 regions of rDNA BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science. ___________________________ Dan E. Krane, Ph.D. ___________________________ David Goldstein, Ph.D. Department Chair, Committee on Biological Sciences Final Examination ____________________________ Dan E. Krane, Ph.D. ____________________________ Stephanie A. Smith, Ph.D. ____________________________ Yvonne M. Vadeboncoeur, Ph.D. ____________________________ Michele Wheatly, Ph.D. Dean, College of Science and Mathematics ____________________________ Joseph F. Thomas, Jr., Ph.D. Dean, School of Graduate Studies ABSTRACT Sharma, Monita. M.S., Department of Biological Sciences, Wright State University, 2007. Molecular Characterization of Chironomid species and their use as bio-indicators. Of all major aquatic invertebrate groups, members of family Chironomidae are most abundant and show a wide range of habitat preferences. The importance of correct identification of Chironomids has been realized in many bioassessment studies mainly because of their worldwide distribution, substrate specificities and predictable responses to various pollutants in the water sources. This study establishes that the sequence data from the Intergenic Spacer Regions (ITS) of ribosomal DNA could be used as molecular markers to distinguish between different Chironomidae species and also to identify them. -

De Eurasian Lakes with Respect to Temperature and Continentality: Development and Application of New Chironomid-Based Climate-Inference Models in Northern Russia
Quaternary Science Reviews 30 (2011) 1122e1141 Contents lists available at ScienceDirect Quaternary Science Reviews journal homepage: www.elsevier.com/locate/quascirev The distribution and abundance of chironomids in high-latitude Eurasian lakes with respect to temperature and continentality: development and application of new chironomid-based climate-inference models in northern Russia A.E. Self a,b,*, S.J. Brooks a, H.J.B. Birks b,c,d, L. Nazarova e, D. Porinchu f, A. Odland g, H. Yang b, V.J. Jones b a Department of Entomology, Natural History Museum, Cromwell Road, London SW7 5BD, UK b Environmental Change Research Centre, Department of Geography, University College London, Gower Street, London WC1E 6BT, UK c Department of Biology, Bjerknes Centre for Climate Research, University of Bergen, P.O. Box 7803, N-5020 Bergen, Norway d School of Geography and the Environment, University of Oxford, South Parks Road, Oxford OX1 3QY, UK e Alfred Wegener Institute for Polar and Marine Research, Telegrafenberg A 43, 14473 Potsdam, Germany f Department of Geography, The Ohio State University, 1036 Derby Hall, 154 N. Oval Mall, Columbus, OH 43210, USA g Institute of Environmental Studies, Telemark University College, N-3800 Bø, Norway article info abstract Article history: The large landmass of northern Russia has the potential to influence global climate through amplification Received 10 June 2010 of climate change. Reconstructing climate in this region over millennial timescales is crucial for Received in revised form understanding the processes that affect the global climate system. Chironomids, preserved in lake 18 January 2011 sediments, have the potential to produce high resolution, low error, quantitative summer air temperature Accepted 19 January 2011 reconstructions.