Turkish Guideline for Atopic Dermatitis 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22 Asteatotic Eczema (Xerosis, Xerotic Eczema, Eczema Craquelé, Eczema Cannalé, Eczema Hiemalis, Winter Itch)
22 Asteatotic Eczema (Xerosis, Xerotic Eczema, Eczema Craquelé, Eczema Cannalé, Eczema Hiemalis, Winter Itch) INTRODUCTION This common dermatitis is often misdiagnosed and usually overtreated. Familiarity with the physical findings will allow an accurate assessment of the underlying cause, and symptoms can usually be corrected with simple measures. The condition occurs for a number of reasons, especially the following: 1. With age, skin sebum secretion diminishes, as does the water-holding capacity of the epidermis. These changes are particularly marked on the lower extremities. 2. Bathing further depletes the epidermis of its water-retaining constituents. 3. Climate has a major effect, and most patients experience symptoms for the first time during a winter season as their skin dries from exposure to the low indoor humidity produced as buildings are heated against inclement weather. Incidence will vary from place to place, depending on the severity of the season and the overall regional weather. CLINICAL APPLICATION QUESTIONS In the early spring, a 75-five-year-old woman visits your office with a complaint of generalized itching. The symptoms began in late December on local skin areas, and have progressed throughout the winter. You suspect an asteatotic eczema. 1. What information from her history may help support your suspicions? 2. What are the primary lesions in areas of asteatotic eczema? 3. What are the secondary lesions seen in asteatotic eczema? 4. What typical configurations strongly support your suspicions? 5. This woman has minimal physical findings, and some provoking factors are evi- dent in her history, but she fails to improve with treatment. What should be done next? APPLICATION GUIDELINES Specific History Onset Symptoms usually are noted in the fifth and sixth decades of life for the first time. -

Japanese Guidelines for Atopic Dermatitis 2020*
Allergology International 69 (2020) 356e369 Contents lists available at ScienceDirect Allergology International journal homepage: http://www.elsevier.com/locate/alit Invited Review Article Japanese guidelines for atopic dermatitis 2020* * Norito Katoh a, , 1, Yukihiro Ohya b, 1, Masanori Ikeda c, Tamotsu Ebihara d, Ichiro Katayama e, Hidehisa Saeki f, Naoki Shimojo g, Akio Tanaka h, Takeshi Nakahara i, Mizuho Nagao j, Michihiro Hide h, Yuji Fujita g, Takao Fujisawa k, Masaki Futamura l, Koji Masuda a, Hiroyuki Murota m, Kiwako Yamamoto-Hanada b, Committee for Clinical Practice Guidelines for the Management of Atopic Dermatitis 2018, The Japanese Society of Allergology, The Japanese Dermatology Association a Department of Dermatology, Kyoto Prefectural University of Medicine Graduate School of Medical Science, Kyoto, Japan b Allergy Center, National Center for Child Health and Development, Tokyo, Japan c Department of Pediatric Acute Medicine, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama, Japan d Department of Dermatology, Keio University School of Medicine, Tokyo, Japan e Department of Dermatology, Graduate School of Medicine, Osaka University, Suita, Japan f Department of Dermatology, Graduate School of Medicine, Nihon Medical School, Tokyo, Japan g Department of Pediatrics, Graduate School of Medicine, Chiba University, Chiba, Japan h Department of Dermatology, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan i Division of Skin Surface Sensing, Department -

Seborrheic Dermatitis
432 Teams Dermatology Done by: Wael Al Saleh & Abdulrahman Al-Akeel Reviewer: Wael Al Saleh & Abdulrahman Al-Akeel 9 Team Leader: Basil Al Suwaine Color Code: Original, Team’s note, Important, Doctor’s note, Not important, Old teamwork 432 Dermatology Team Lecture 9: Atopic dermatitis/ Eczema Objectives 1- To know the definition & classification of Dermatitis/Eczema 2- To recognize the primary presentation of different types of eczema 3- To understand the possible pathogenesis of each type of eczema 4- To know the scheme of managements lines P a g e | 1 432 Dermatology Team Lecture 9: Atopic dermatitis/ Eczema Introduction: A groups and spectrum of related disorders with pruritus being the hallmark of the disease, they also come with dry skin. Every atopic dermatitis is eczema but not every eczema are atopic dermatitis. Atopic dermatitis mean that the patient has eczema (excoriated skin, itching and re-onset) and atopy (atopy; the patient or one of his family has allergic rhinitis, asthma or eczema). It starts early of life (eczema can happen at any time). It classified as: - Acute, characterized by erythema, papules, vesicles, oozing, and crusting. - Subacute, clinically it is represented by erythema, scaling, and crusting. - Chronic, presents with thickening of the skin, skin markings become prominent (lichenification); pigmentation and fissuring of the skin occur. Acute on top of chronic very dry 4 years old boy with chronic, itchy, well defined brownish plaque with bleeding plaques. lichenifications. Ill defined plaques Well defined erythematous excoriated Lichenification is the hallmark for plaques on both cheeks with erosion. chronic course. P a g e | 2 432 Dermatology Team Lecture 9: Atopic dermatitis/ Eczema Dermatitis Classification of dermatitis: Atopic, more common in children Seborrheic (oily skin)- (like naso-labial folds, scalp, ears) Contact dermatitis, substance cause eczema - Allergic - Irritant Nummular, coined shape, usually in the shin. -

Pathophysiology and Treatment of Pruritus in Elderly
International Journal of Molecular Sciences Review Pathophysiology and Treatment of Pruritus in Elderly Bo Young Chung † , Ji Young Um †, Jin Cheol Kim , Seok Young Kang , Chun Wook Park and Hye One Kim * Department of Dermatology, Kangnam Sacred Heart Hospital, Hallym University, Seoul KS013, Korea; [email protected] (B.Y.C.); [email protected] (J.Y.U.); [email protected] (J.C.K.); [email protected] (S.Y.K.); [email protected] (C.W.P.) * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Pruritus is a relatively common symptom that anyone can experience at any point in their life and is more common in the elderly. Pruritus in elderly can be defined as chronic pruritus in a person over 65 years old. The pathophysiology of pruritus in elderly is still unclear, and the quality of life is reduced. Generally, itch can be clinically classified into six types: Itch caused by systemic diseases, itch caused by skin diseases, neuropathic pruritus, psychogenic pruritus, pruritus with multiple factors, and from unknown causes. Senile pruritus can be defined as a chronic pruritus of unknown origin in elderly people. Various neuronal mediators, signaling mechanisms at neuronal terminals, central and peripheral neurotransmission pathways, and neuronal sensitizations are included in the processes causing itch. A variety of therapies are used and several novel drugs are being developed to relieve itch, including systemic and topical treatments. Keywords: elderly; ion channel; itch; neurotransmission pathophysiology of itch; pruritogen; senile pruritus; treatment of itch 1. Introduction Citation: Chung, B.Y.; Um, J.Y.; Kim, Pruritus is a relatively common symptom that anyone can experience at any point in J.C.; Kang, S.Y.; Park, C.W.; Kim, H.O. -

The Art of Diagnosis
Dermatology elective for yr. 5 Natta Rajatanavin , MD. Div. of dermatology Dep. Of Medicine , Ramathibodi Hospital Mahidol University 23rd Feb 2015 How to diagnosis and manage eczema and psoriasis. Objectives • Identify and describe the morphology of eczema and psoriasis • Describe associated triggers or risk factors for eczema and psoriasis • Describe the clinical features of psoriatic arthritis • List the basic principles of treatment for eczema and psoriasis Approach dermatologic disease with an understanding of basic skin structure and microanatomy Can you name the four major layers of the epidermis? Stratum corneum Stratum granulosum (granular cell layer) Stratum spinosum (spiny layer) Stratum basale (basal cell layer) 6 Scale/scale crust Eczema/dermatitis Layers of the skin Epidermis Below the dermis lies fat, also called Dermis subcutis, panniculus, or Subcutis hypodermis. 10 Erythema nodosum Eczema/ dermatitis No.1 common skin problem Most common symptom is pruritus Eczema/dermatitis is a immunologic reaction of our skin to antigens Langerhans Cells important in the induction of delayed-type hypersensitivity 14 Classification of eczema Exogenous • Allergic • Photo allergic dermatitis • Irritant Endogenous • Skin barrier defect • sequence of histological events in eczema after contact with antigen. Eczema; acute, sub-acute stage Subacute eczema Subacute eczema histology Eczema; chronic stage Lichenfication: lichen simplex chronicus lichen simplex chronicus histology Classification of Exogenous eczema • Allergic contact dermatitis -

Revista ABD Volume 92 Numero 4
Particular characteristics of atopic eczema in tropical environments.INVESTIG The TropicalA Environment...TION 671671 s Xerosis cutis and associated co-factors in women with prurigo nodularis* Sevgi Akarsu1, Ozlem Ozbagcivan1, Turna Ilknur1, Fatma Semiz1, Burcu Bahar Inci1, Emel Fetil1 DOI: http://dx.doi.org/10.1590/abd1806-4841.20187127 Abstract: BACKGROUND: Current data regarding the associated factors of prurigo nodularis are still uncertain, except for atopic predisposition. OBJECTIVES: The purposes of this study were to (1) determine the frequencies of xerosis and other accompanying diseases of female patients with prurigo nodularis; (2) compare the demographic, clinical and accompanying disease characteristics by grouping these patients according to whether they have associated xerosis (who were subsequently subgrouped as atopic or non-atopic) or not. METHODS: In this retrospective descriptive study, 80 females with PN were categorized according to the accompanying diseases (dermatological, systemic, neurological, psychogenic, mixed, or undetermined origin). RESULTS: A total of 45 associated co-factors including dermatological in 63 (78.8%), systemic in 57 (71.3%), psychological in 33 (41.3%) and neurological co-factors in 14 (17.5%) of all patients with prurigo nodularis were detected. Xerosis was observed in 48 (60%) patients (non-atopic co-factors in 66.7% of them). The ratio of patients with mixed co-factors, dermatological+sys- temic co-factors and dermatological+systemic+psychological co-factors were found to be significantly higher in patients with xerosis compared to those without xerosis. STUDY LIMITATIONS: Our study has certain limitations such as the absence of an age-matched control group, absence of follow-up data and the fact that the diagnosis of xerosis has not been based on objective methods. -

B K B Ld I MD Brooke Baldwin, MD Private Practice, Lutz, Florida Chief
BBkrooke BBldialdwin, MD Private Practice, Lutz, Florida Chief of Dermatology James A Haley VA Hospital Adjunct Assistant Professor of Dermatology University of Florida What we are going to cover today Dermatologic Emergencies Common benign skin growths Malignant skin tumors Common Rashes Photoprotecti on and CiCosmetics Dermatologic Emergencies Erythroderma PlPustular psoriiiasis Pemphigus DRESS Syndrome SJS / TEN EEthdrythroderma Erythroderma Generalized redness and scaling of skin involving >90% BSA Systemic manifestations PihPeripheral edema & ffilacial edema Tachycardia Loss of fluids and proteins Disturbed thermoregulation Most common etiologies Atopic dermatitis, psoriasis, CTCL, drug reactions Despite intensive evaluation, the cause remains unknown in 25‐30% PPlustular psoriiiasis Pustular Psoriasis Generalized pustular psoriasis Unusual mani festation o f psoriasis Triggering factors Pregnancy (impetigo herpetiformis) Tapering of corticosteroids (Von Zumbusch reaction) HliHypocalcemia Infections Topical irritants Rarely treatment with TNF alpha blockers (palms and soles) Pemphigus Group of chronic autoimmune blistering diseases presenting with painful erosions IgG Autoantibodies are directed against the cell surface of keratinocytes Results in blistering in varying areas of the epidermis Diagnosi s is confirmed wihith direct iflimmunofluorescence on skin biopsy 3 ma jor forms P. vulgaris, P. foliaceus, paraneoplastic Do not confuse with Bullous pemphigoid which presents with tense bullae Pempgphigus -

Prospective Trial Comparing Topical Steroid Application to Wet Versus
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine January 2014 Prospective Trial Comparing Topical Steroid Application To Wet Versus Dry Skin In Children With Atopic Dermatitis Lucinda Shuangyuan Liu Yale School of Medicine, [email protected] Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Liu, Lucinda Shuangyuan, "Prospective Trial Comparing Topical Steroid Application To Wet Versus Dry Skin In Children With Atopic Dermatitis" (2014). Yale Medicine Thesis Digital Library. 1897. http://elischolar.library.yale.edu/ymtdl/1897 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. 1 PROSPECTIVE TRIAL COMPARING TOPICAL STEROID APPLICATION TO WET VERSUS DRY SKIN IN CHILDREN WITH ATOPIC DERMATITIS A Thesis Submitted to the Yale University School of Medicine In Partial Fulfillment of the Requirements for the Degree of Doctor of Medicine by Lucinda Shuangyuan Liu 2014 2 ABSTRACT PROSPECTIVE TRIAL COMPARING TOPICAL STEROID APPLICATION TO WET VERSUS DRY SKIN IN CHILDREN WITH ATOPIC DERMATITIS. Lucinda S. Liu, Yanna Kang, and Richard J. Antaya. Department of Dermatology, Yale University School of Medicine, New Haven, CT. The aim of this study was to determine whether “soak and smear,” a technique where hydration via a 10-minute soak in lukewarm plain water followed by topical corticosteroid application to wet skin, is efficacious for the treatment of atopic dermatitis in pediatric patients. -
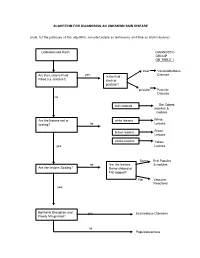
ALGORTIHM for DIAGNOSING an UNKNOWN SKIN DISEASE (Note
ALGORTIHM FOR DIAGNOSING AN UNKNOWN SKIN DISEASE (note: for the purposes of this algorithm, consider purple as red lesions and blue as brown lesions) Undetermined Rash DIAGNOSTIC GROUP ON TABLE 1 clear Vesiculobulbous yes Disease Are the Lesions Fluid Is the fluid Filled (i.e. blisters?) clear or pustular? pustular Pustular Disease no skin colored Skin Colored papules & nodules Are the lesions red or white lesions White scaling? no Lesions brown lesions Brown Lesions yellow lesions Yellow yes Lesions Dome Red Papules no Are the lesions & nodules Are the lesions Scaling? Dome-shaped or Flat-topped? Flat Vascular Reactions yes Epithelial Disruption and yes Eczematous Diseases Poorly Marginated? no Papulosquamous TABLE-1 COMMON DIAGNOSES FOR EACH GROUP A) Vesiculobulbous Diseases Vesicular: - Herpes Simplex - Herpes Zoster - Varicella - Vesicular Tinea Pedis - Dyshidrosis - Contact Dermatitis Bullous: - Stevens-Johnson Syndrome - Pemphigus Vulgaris - Bullous Pemphigoid - Bullous Impetigo - Trauma (burns, for e.g.) Hemorrhagic Bullae: - Necrotizing Fasciitis - Vasculitis - Bullous Pyoderma Gangrenosum - Envenomation B) Pustular Diseases True Pustules: - Bacterial Folliculitis - Fungal Folliculitis - Candidiasis - Acne Rosacea - Pustular Psoriasis Pseudo or Solid "Pustules": - Acne - Milia - Keratosis Pilaris C) Skin Colored Papules & Nodules Actinic Keratoses Squamous Cell Carcinoma Seborrheic Keratosis Basal Cell Carcinoma Warts (plantar, genital, etc.) Epidermoid Cysts Dermal Nevi Molluscum Contagiosum Skin Tags D) White Lesions Patches -

Clinical Study on Dermatological Presentation in 100 Cases of Hiv Infection
CLINICAL STUDY ON DERMATOLOGICAL PRESENTATION IN 100 CASES OF HIV INFECTION DISSERTATION Submitted To the Tamilnadu Dr.M.G.R. Medical University In Partial Fulfilment Of The Requirements For The Award Of Degree Of M.D. BRANCH XІІ A (Dermatology, Venereology and Leprosy ) DEPARTMENT OF DERMATOLOGY, VENEREOLOGY & LEPROSY COIMATORE MEDICAL COLLEGE COIMBATORE-641014 THE TAMILNADU DR.M.G.R.MEDICAL UNIVERSITY CHENNAI – TAMILNADU APRIL – 2011 CERTIFICATE This is to certify that this dissertation entitled “CLINICAL STUDY ON DERMATOLOGICAL PRESENTATION IN 100 CASES OF HIV INFECTION” is a bonafide work done by DR. LINCY C.F, Post Graduate in M.D. Dermatology, Venereology and Leprosy, Coimbatore Medical College, Coimbatore- 641014, during the academic year 2009-2010. This work was done under my direct guidance and supervision and submitted for the M.D.BRANCH XІІ A examination in April 2011 to the Tamil Nadu Dr.M.G.R. Medical University, Chennai. Dr.R.VIMALA, M.D. Dr.P.P. RAMASAMY, M.D., D.D., DEAN, Professor and Head of the Department, Coimbatore Medical College, Department of Dermatology & Leprosy, Coimbatore- 641014 Coimbatore Medical College, Coimbatore - 641014 DECLARATION I Dr.LINCY C.F, solemnly declare that this dissertation titled “CLINICAL STUDY ON DERMATOLOGICAL PRESENTATION IN 100 CASES OF HIV INFECTION” is a bonafide work done by me at Coimbatore Medical College during 2009-2010 under the guidance and supervision of Prof. Dr.P.P.Ramasamy, M.D., D.D., Professor and Head, Department of Dermatology, Coimbatore Medical College, Coimbatore- 641014. This dissertation is submitted to The Tamilnadu Dr. M.G.R Medical University, towards partial fulfilment of requirement for the award of M.D. -

Atopic Dermatitis in Adults
Chapter Atopic Dermatitis in Adults: Epidemiology, Risk Factors, Pathogenesis, Clinical Features, and Management Olumayowa Abimbola Oninla, Ayesha Omolara Akinkugbe, Bolaji Ibiesa Otike-Odibi, Mufutau Muphy Oripelaye and Fatai Olatunde Olanrewaju Abstract Atopic dermatitis (AD) is an itchy chronic relapsing inflammatory skin condition mostly affecting children than adults. Eczematous conditions are com- mon worldwide with increase in the prevalence in both developed and developing countries. AD in adults is of two types – the first type starts as AD in childhood and gradually progresses to adulthood (Persistent AD) and the second type results from AD developing in adulthood (Adult-onset AD). The article reviews and discusses this condition in adults considering the epidemiology, risk factors, pathogenesis, diagnostic criteria, and management of this condition. Keywords: Atopic dermatitis, Adult, Adult-onset Atopic dermatitis, Eczema 1. Introduction Atopic dermatitis (AD) is an itchy chronic relapsing inflammatory skin condi- tion mostly affecting children than adults with atopy. Atopy was derived from the Greek word “atopos” by Coca and Cooke in 1923 for the grouping of asthma, hay fever and asthma. [1] In an article by Kanwar, atopic dermatitis (AD) or atopic eczema was defined as “an itchy, inflammatory skin condition characterized by poorly defined erythema with edema, vesicles, and weeping in the acute stage and skin thickening (lichenification) in the chronic stage,” [2] and in the year 2000, Bannister and Freeman originated the term adult-onset atopic dermatitis for the condition in adulthood. [3] AD usually occur as a continuum of childhood AD but few cases start in adulthood, hence, the term - Adult onset AD. AD in adults is of two types – the first type occurs as AD in childhood and progresses to adulthood condition (Persistent AD) while the second type results from AD developing in adulthood (Adult-onset AD). -

Healthcare Bulletin
Happy New Year 2003 JANUARY 2003 VOL 11 NO 1 ISSN 1681-5552 healthcare bulletin ◆ Common Dermatological Disorders ◆ Eczema ◆ Pruritus ◆ Urticaria ◆ Product Profile: Oni® ◆ Glimpse of MSD Activities 2002 ◆ SQUARE in International Business ◆ Medical Updates Published as a service to medical professionals by JANUARY 2003 VOL 11 NO 1 IN THIS ISSUE: Common Dermatological Disorders ... Page 1 Eczema ... Page 3 Pruritus ... Page 7 Urticaria ... Page 12 Product Profile: Oni® ... Page 16 Glimpse of MSD Activities 2002 ... Page 17 From the Desk of Managing Editor SQUARE in International Business ... Page 19 Dear Doctor: Medical Updates ... Page 20 "! You have already noticed Greetings everyone and Happythe New SQUARE Year! Welcome to this edition of " the new look of this issue! We have updated our design to make it more enjoyable for you. Our vision and determination is to give you the most accurate, " is "Dermatology" special and includes reliable andthe easy SQUARE to understand health information every time. This “the SQUARE” issue of " updated information on common dermatological disorders, Managing Editor pruritus, eczema, urticaria. Besides, our regular features comprise, Omar Akramur Rab MBBS, FCGP, FIAGP, FRSH medical updates, product profile and others. Executive Editor We welcome your suggestions and comments to help us provide Latifa Nishat the highest quality and most useful service. In addition we MBBS appreciate all of the comments and feedback we have received Members of the Editorial Board from those who have taken the time to write. Muhammadul Haque We believe you will enjoy reading this publication and that MBA hope and A. H. Mahbub Alam the contents provided will prove helpful towardsSQUARE your goal of M Pharm, PhD optimum health! Shaokat Zaman MBBS We, on behalf of the management of Information Assistance pray that you have a safe and healthy life throughout all of Md.