Pulsed Current Electrodeposition of Silicon Thin Films Anodes for Lithium Ion Battery Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHENNAI Provisionally Selected Candidates (General)
School Name: KV MADURAI NO 1 Region: CHENNAI List 2 (09-04-2018) Provisionally Selected Candidates (General) as on 09-04-2018 Number Serial Middle Service Enrolment ID First Name Last Name of Numbe Name Category Transfers r 280148950118 JALADHIJA P 1 3 1 280148995398 DHARUN VARAHITH 1 3 2 280148938283 C SRI DAKSHAYA 1 3 3 280149136073 NIKETH S RAJ 1 3 4 280148977886 MADHU MITHRA V 1 3 5 280149197336 R KAARNIKA 1 3 6 280148982530 A. ABHINAVSHREE 1 3 7 280149174910 MITHUN B 1 3 8 280148982746 ERIN SHANU 1 3 9 280149057203 MANOPRATHAB 1 3 10 280149148218 VISVAK R 1 3 11 280149286581 A SAIDHARSHAN 1 3 12 280148924863 K PAVITHRA 1 3 13 280148960307 ISHIT VATSYAYAN 1 3 14 280149095450 B DIYA 1 3 15 280149075872 LAKSHAYA A 1 3 16 280149119847 A PUNITHA 1 3 17 280149183131 KANISHA S 1 3 18 280148941730 M MANOJKUMAR 1 3 19 280149036593 TEJASHVINI R 1 3 20 280148900445 DHARSHINI R 1 3 21 280148954191 V. HASHVANTH 1 3 22 280149066575 Y MOUTHIKA VAISHNAVI 1 3 23 280149089166 SADHAN SANKESH K 1 2 24 280148960020 M JAYABADRI 1 2 25 280149089360 THIRUMALAI SELVAN R 1 2 26 280148905385 T AKSHITHA 1 2 27 280148940732 INBHA PRAJEN J 1 2 28 280149345033 VIDYUT U 1 2 29 280149133559 DHAANIYA D 1 2 30 280149177574 I MONISHA 1 2 31 280149040822 SUBHIKSHA J C 1 2 32 280149243872 N.KOYAL 1 2 33 280149350480 SEEMATH DURAI T 1 2 34 280149273917 SANJANA B 1 2 35 280149218775 DHARSHANA J 1 2 36 280149273881 SHOOBRIT HARI R 1 2 37 280148929655 CHARLIE IRUDHAYARAJ 1 2 38 280148919974 DHIYANESSVAR M 1 2 39 Wait Listed Candidates (General) as on 09-04-2018 - PAGE 1 Number Serial Middle Service Enrolment ID First Name Last Name of Numbe Name Category Transfers r 280148932898 ROHIT R K 1 2 1 280148900297 HARSHINI SAAI AARADHYA S.K 1 2 2 280148926766 C. -

ANNUAL REPORT June 2016 – May 2017
MOTHER TERESA WOMEN’S UNIVERSITY KODAIKANAL ANNUAL REPORT June 2016 – May 2017 Edited by : Dr.A.Muthu Meena Losini Assistant Professor Department of English Mother Teresa Women‘s University Kodaikanal Technical Assistance by : Mrs.T.Amutha Technical Assistant Department of Physics Mother Teresa Women‘s University Kodaikanal MOTHER TERESA WOMEN‟S UNIVERSITY (Accredited with „B‟ Grade by NACC) KODAIKANAL – 624 101 This is the Annual Report for the period June 2016 – May 2017 of the Mother Teresa Women‘s University, Kodaikanal. The report gives all Academic, Administrative and other relevant details pertaining to the period June 2016 – May 2017. I take this occasion to thank all the members of the Executive Council, Academic Committee and Finance Committee for all their participation and contribution to the growth and development of the University and look forward to their continued advice and guidance. Dr. G. Valli VICE-CHANCELLOR INTRODUCTION The Executive Council has great pleasure in presenting to the Academic Committee, the Annual report for the period June 2016- May 2017 as per the requirement of the section 26 of Chapter IV of Mother Teresa Women‘s University Act 1984. The report outlines the various activities which have paved for the growth and development of the University through the period June 2016 –May 2017 in the areas of Teaching, Research and Extension; it provides a detailed account of various Academic Achievements, Introduction of new Innovative Courses, Conduct of Seminar/ Workshop/ Conferences at Regional, National and International levels and its extension activities. OFFICERS AND AUTHORITIES OF THE UNIVERSITY CHANCELLOR - HIS EXCELLENCY HON‘BLE Banwarilal Purohit Governor of Tamil Nadu PRO – CHANCELLOR - HONOURABLE Thiru. -

Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications
coatings Review Porous Zinc Oxide Thin Films: Synthesis Approaches and Applications Marco Laurenti * and Valentina Cauda Department of Applied Science and Technology, Politecnico di Torino, C.so Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-011-090-7394 Received: 17 December 2017; Accepted: 7 February 2018; Published: 9 February 2018 Abstract: Zinc oxide (ZnO) thin films have been widely investigated due to their multifunctional properties, i.e., catalytic, semiconducting and optical. They have found practical use in a wide number of application fields. However, the presence of a compact micro/nanostructure has often limited the resulting material properties. Moreover, with the advent of low-dimensional ZnO nanostructures featuring unique physical and chemical properties, the interest in studying ZnO thin films diminished more and more. Therefore, the possibility to combine at the same time the advantages of thin-film based synthesis technologies together with a high surface area and a porous structure might represent a powerful solution to prepare ZnO thin films with unprecedented physical and chemical characteristics that may find use in novel application fields. Within this scope, this review offers an overview on the most successful synthesis methods that are able to produce ZnO thin films with both framework and textural porosities. Moreover, we discuss the related applications, mainly focused on photocatalytic degradation of dyes, gas sensor fabrication and photoanodes for dye-sensitized solar cells. Keywords: zinc oxide; thin films; high surface area; porous structure; sputtering; electrodeposition; spray pyrolysis; template-assisted methods 1. Introduction Zinc Oxide (ZnO) is a well-known metal oxide material showing interesting biocompatible [1,2], semiconducting [3], optical [4], photocatalytic [5], resistive switching [6] and piezoelectric properties [7]. -

Author Index 399 Sunday, 02:30 PM - 04:00 PM Aigbedo, Henry
Author Index 399 Sunday, 02:30 PM - 04:00 PM Aigbedo, Henry . Afeche, Philipp 344 Sunday, 08:00 AM - 09:30 AM ., Lindawati 518 38 Friday, 09:45 AM - 11:15 AM Monday, 09:45 AM - 11:15 AM 193 Saturday, 08:00 AM - 09:30 AM Aflaki, Arian Akabane, Getulio 385 Sunday, 09:45 AM - 11:15 AM 75 Friday, 01:30 PM - 03:00 PM 145 Friday, 05:00 PM - 06:30 PM 356 A 237 Saturday, 09:45 AM - 11:15 AM Sunday, 08:00 AM - 09:30 AM 370 Sunday, 09:45 AM - 11:15 AM Aflaki, Sam Abbasi, Babak 446 Sunday, 04:15 PM - 05:45 PM 133 Friday, 03:15 PM - 04:45 PM 452 75 Friday, 01:30 PM - 03:00 PM Sunday, 04:15 PM - 05:45 PM 169 Friday, 05:00 PM - 06:30 PM 477 Monday, 08:00 AM - 09:30 AM Abbey, James 339 Sunday, 08:00 AM - 09:30 AM 528 Monday, 09:45 AM - 11:15 AM 99 Friday, 01:30 PM - 03:00 PM Agarwal, Akshit 560 Monday, 11:30 AM - 01:00 PM 122 Friday, 03:15 PM - 04:45 PM 5 Friday, 08:00 AM - 09:30 AM Akartunali, Kerem Abdala, Etienne Agarwal, Anurag 54 Friday, 09:45 AM - 11:15 AM 526 Monday, 09:45 AM - 11:15 AM 441 Sunday, 04:15 PM - 05:45 PM Akasaka, Shingo Abdi, M Reza 551 Monday, 11:30 AM - 01:00 PM 378 Sunday, 09:45 AM - 11:15 AM 89 Friday, 01:30 PM - 03:00 PM Agarwal, Atul Akcali, Elif 411 Sunday, 02:30 PM - 04:00 PM 338 Sunday, 08:00 AM - 09:30 AM 50 417 Sunday, 02:30 PM - 04:00 PM Friday, 09:45 AM - 11:15 AM 505 Monday, 09:45 AM - 11:15 AM Aghajani, M. -

C/O Lalitha - # 76, 3Rd Cross - 5Th Main Road, 3Rd Cross, 'C' - VI Main, V Cross, Opp
R2005 F2009 MYS/14/MAR R 2009 MYS/27/NOV CR0327643/01/2005 310158596/03/2009 Abdul Haleem A K MANI Aadil A. - # 53 - C/o Lalitha - # 76, 3rd Cross - 5th Main Road, 3rd Cross, 'C' - VI Main, V Cross, Opp. Dharwad - 3rd Main, 'A' Layout, C.V. Road Lauout Bakery - Bannimantap - Bannimantap - Saraswathipuram - Mysore 570015 - Mysore. 570015 - Mysore 570009 0821 2497244 9945980994 9739459326 [email protected] [email protected] L2009 MYS/01/FEB F2009 MYS/02/DEC L2009 MYS/21/FEB 320334261/08/2005 Abel Mathew Zacharia H. Abhijit Ginimav Abhijith C. - Poozhikalayil House - Aeradhita Apartment - # 1448/49, 8th Cross - Muttambalam (P), Eqanjal - Bhavane Nagar - Srirampura 2nd Stage - Kottayam - Keshwupur - - Kerala - Hubli - Mysore 570023 821 2361426 9742242411 9886912119 9986700338 [email protected] [email protected] [email protected] R2009 MYS/06/MAR F2008 MYS/17/SEP R2008 MYS/05/AUG 320491612/03/2009 310150459/10/2008 320442450/08/2008 Abhijith C. N. ABHILASH M S ABHILASH U - # 355, Gurukar Devanna Street - #260 - #145, Ground Floor, 2nd Main - Old Agrahara Fort Mohalla - 5th Main - 2nd Stage - - Brindavan Extension - Gokulam - Mysore 570004 - Mysore 570020 - Mysore 570002 821 2414179 821 9986157784 9844599752 9740596316 [email protected] [email protected] [email protected] R2009 MYS/38/MAY F2009 MYS/11/JUNE F2009 MYS/19/MAR 320501841/05/2009 310165184/06/2009 31015854903/2009 Abhilash M. K. Abhilasha Abhilasha M. - # 1036/10B, 1st Main, 1st Road - D/o Govinda Shetty - # 299, 4th Main - S.J.H. Road - # B-10, AIISh Quatres - 9th Cross, 'D' Block - Vidyaranaya Puram - Manasagangothri - Ramakrishna Nagar - Mysore 570008 - Mysore 570006 - Mysore 570023 9035238885 9242919724 9342958426 [email protected] [email protected] F 2007 MYS/038/Sept. -

Nrityagram Dance Ensemble & Chitrasena Dance Company
Nrityagram Dance Ensemble & chitrasena dance company: A Dance Village Odyssey Study Guide PreK- Grade 12 TABLE OF CONTENTS Theatre Etiquette 3 Nrityagram Dance Village 4 Nrityagram Dance Ensemble 5 Surupa Sen & Pavithra Reddy 6 About India 7 Hinduism 8 The History of OdissI 9-10 Odissi Technique 11 Bhangas 12 Mudras/Hastas 13 Bedhas 14 Costumes 15 Music 16 Vocabulary 17 Sri Lanka 18 Kandy 19 Kandyan Dance 19 Other Types of Kandyan Dance, Costumes, Music, Kandyan Dance Today 20 Chitrasena Dance Company 21 Activities 22-25 Grades PreK-3 22 Grades 4-6 22-23 Grades 7-8 23-24 Grades 9-10 24-25 Grades 11-12 25 Resources 26 Credits 27 “When you know what you want, the entire universe conspires with you to realize it.” - Protima Gauri (1948-1998), Founder of Nrityagram Dance Ensemble & Village 2 Theatre Etiquette Audience members are an important and unique part of live theater. Remember that anything you say or do as an audience member will be visible and audible to the performer onstage. Your behavior as a member of the audience will make this performance memorable and enjoyable for everyone. Remember to keep these things in mind: ❖ Leave your food, drinks, and gum in your classroom. ❖ Turn off all electronics. An unexpected beep or ring in the middle of the performance will distract the audience members and the performers! ❖ Remember to respond appropriately and at the correct times. Make sure you pay attention at all times, to let the performer know you are engaged in their performance. They will also let you know what is appropriate and what is not. -

Kongu Engineering College. Campus R&D News
6. Dr.R.Selvakarthi, Electronics and Instrumentation Engineering and KONGU ENGINEERING COLLEGE. Dr.P.Shanmugam, Chemical Engineering received research grant of Rs.2,43,000/- for mentoring the industry project entitled Waterless dyeing CAMPUS R&D NEWS machine from EDII under IVP scheme on 27.07.2020, Ref.No. Lr.No.2270/EDII/JD/2018 7. Dr.M.Karthick, Dr.S.Usha and P.Karthikeyan of Electrical and Electronics VOL 01 DECEMBER 2020 ISSUE 12 Engineering received research grant of Rs.18,66,667/- for the project entitled An investigation into the maneuvering performance of Proton Exchange Membrane (PEM) fuel cell for its recoverable degradation of electrical behavior under Ph.D Viva-voce completed extreme humidification and membrane hydration conditions from AICTE under 1. P.Vidhyalakshmi, Research Scholar, Department of Electronics and RPS scheme on 14.08.2020, Ref. No.8-55/FDC/PRS(POLICY-1)/2019-20. Instrumentation Engineering defended her thesis entitled Computer vision based 8. R.P.Karthick of Electronics and Communication Engineering received research system for localizing structural defects in textile web material using texture grant of Rs.2,65,000/- for mentoring the industry project entitled Intelligent analysis techniques on 04.12.2020. assistive device for people with vision impairment from EDII under IVP scheme on 29.08.2020, Ref.No. Lr.No.2270/EDII/JD/2018 9. Dr.N.Senthilnathan and P.Sethupathi of Electrical and Electronics Engineering R&D PROJECTS SACTIONED (2020-2021) received research grant of Rs.6,79,000/- for the project entitled Design and 1. Dr.R.Baskar, Food Technology, Dr.C.Maheswari, Mechatronics Engineering and development of novel submersible line start permanent magnet assisted Dr.T.Manikandan, Chemistry received research grant of Rs.10,11,137/- for the synchronous reluctance motor for agricultural water pumping from DST under project entitled Developing electrical discharge non-thermal plasma (EDNP) TDT scheme on 30.09.2020, Ref.No.DST/TDT/AGRO35/2020 (G). -
Papanasam Shivan Krithis Contents
Papanasam Shivan Krithis “Papanasam Sivan was born in 1890 in Polagam near Thanjaavoor to Sri Ramamritha Iyer and Yogambal. He was called Ramaiah by people close to him. The Papanasam of Sri Sivan is between Kumbakonam and Thanjavoor, with its own railway station served by passenger trains. That is where his brother stayed and he used to pass much of his time there. From 1898 to 1910 he lived in Thiruvanthapuram. In 1917 he married Lakshmi, daughter of Sri Ayyaaswami Iyer of Nagapattinam. He also translated many Malayalam and Sanskrit works into Tamil. He passed away in 1973 at the age of 83. " .He is supposed to have written 2000 songs out of which majority were for Tamil films .Experts have identified 400 Krithis which are Carnatic Music Krithis (Info from http://tamilnation.co/culture/music/papanasa_ sivan.htm ) I have translated 42 of them out of which 40 of them are Tamil Krithis and two are Sanskrit Krithis. They are given below Contents Papanasam Shivan Krithis ........................................................................ 1 1. Adbhutha leelaigalai ................................................................ 3 2. Alavillaye .................................................................................. 4 3. Appan Avatharitha ................................................................... 5 4. Devi neeye Thunai .................................................................... 5 5. Durga Lakshmi Saraswathi ....................................................... 6 1 6. Easane indha ........................................................................... -
List of Students from Brilliant Joined MBBS
Brilliant STUDY CENTRE MBBS ADMISSION 2020 - STUDENT STATUS No. Name College & Place 1 A ABHIJITH KOPPAL INSTITUTE OF MEDICAL SCIENCES, KARNATAKA 2 A DYSHERLY ANNET MADRAS MEDICAL COLLEGE, TAMIN NADU 3 A K ABHINAV GOVERNMENT MEDICAL COLLEGE TVM NIMRA INSTITUTE OF MEDICAL SCIENCE (NIMS), VIJAYAWADA, 4 A K FATHIMA FIDHA ANDHRA PRADESH SRI SIDDHARTHA INSTITUTE OF MEDICAL SCIENCE & RESEARCH 5 A KRISHNAVENI CENTER, T BEGUR, KARNATAKA 6 A LAMIA NAZREEN MES MEDICAL COLLEGE, MALAPPURAM 7 A S INDHUJA RAJA MUTHAIAH MEDICAL COLLEGE ANNAMALAI, TAMIL NADU 8 A S M SHAHBAS ASLAM AFMC PUNE NTAIONAL MODERN MEDICAL EDUCATION & RESEARCH INSTITUTE, 9 AADHARSHINI M R DADRA NAGAR HAVELI 10 AADHILEKSHMI A S MBBS ABROAD 11 AADIL JABIR TRAVANCORE MEDICAL COLLEGE KOLLAM 12 AAGAMA SUMESH AIIMS BHUBANESWAR 13 AAKASH MITHRA SREE BALAJI MEDICAL COLLEGE AND HOSPITAL, CHENNAI 14 AALIYA NESRIN E A SREE NARAYANA INSTITUTE OF MEDICAL SCIENCE ERNAKULAM 15 AAMIROSE S RIMS ONGOLE, ANDRAPRADESH 16 AAQIL AHAMED GOVERNMENT MEDICAL COLLEGE ALAPPUZHA 17 AARIF MUHAMMED M GOVERNMENT MEDICAL COLLEGE,TIRUNELVELI 18 AARON MATHEW JOSHUA JUBILEE MISSION MEDICAL COLLEGE THRISSUR 19 AARON S PUTHUSSERY MANDHYA INSTITUTE OF MEDICAL SCIENCE, KARNATAKA 20 AARUSHI NARESH SINGH MYSORE MED& RESEARCH INST MYSORE 21 AARYA S MBBS ABROAD 22 AARYADAS T R KMCT MEDICAL COLLEGE MANASSERY KOZHIKODE 23 AARYAKA J S SREE GOKULAM MEDICAL COLLEGE HOSPITAL TVM 24 AASHLY SAJI KAP VISWANATHAM GMC,TIRUCHIRAPALLI 25 AATHIL SAMAD GOVERNMENT MEDICAL COLLEGE KOLLAM 26 AATIFA BEGUM N TRAVANCORE MEDICAL COLLEGE KOLLAM 27 AAVANI A V GOVERNMENT MEDICAL COLLEGE PALAKKAD 28 AAVANI JAYAN YENEPOYA MEDICAL COLLEGE, KARNATAKA 29 AAYUSH REJI CMC VELLORE 30 ABAAN AKBAR GOVERNMENT MEDICAL COLLEGE ERNAKULAM 31 ABBAS A BACKER AMALA INSTITUTE MEDICAL SCIENCE, THRISSUR 32 ABDUL BASAM D AIIMS BHUBANESWAR 33 ABDUL BASIT PALAYIL THOTTAPPAYIL MADURAI MEDICAL COLLEGE, MADURAI 34 ABDUL BASITH NASER MOHAMED MES MEDICAL COLLEGE, MALAPPURAM 35 ABDUL FATHAH NK GOVERNMENT MEDICAL COLLEGE THRISSUR No. -

Mandatory Disclosure
PSG Institute of Technology and Applied Research Neelambur, Coimbatore – 641 062 Mandatory Disclosure AICTE AICTE File No Southern/ 1-2183174231/ 2014 Period of last approval 2019-20 1.Name of the Institution PSG Institute of Technology and Applied Name of the Institution Research Address of the Institution Avinashi Road, Neelambur City & Pin Code Coimbatore – 641 062 State /UT Tamil Nadu Longitude & Latitude 11.06 N & 77.10 E Phone number with STD code 0422 3933666 Mobile number +91 87540 42808, +91 87540 43808 FAX number with STD code 0422 3933456 Office hours at the Institution 8:30 am to 4:45 pm Academic hours at the Institution 8:30 am to 4:45 pm E-Mail [email protected] Website www.psgitech.ac.in Nearest Railway Station (dist in Km) Coimbatore railway station (16.8 km) Nearest Airport (dist in Km) Coimbatore Airport (8 km) Type of Institution Private-Self Financed Category (1) of the Institution Non Minority Category (2) of the Institution Co-Ed 2. Name and Address of the Trust Name of the Trust running the PSG & Sons’ Charities Institution Type of the Organization Trust Address of the Organization B NO. 1609, Avinashi Road, Peelamedu City & Pin Code Coimbatore – 641 004. State /UT Tamil Nadu Registered with Court of Subordinate Judge, Coimbatore Phone Number with STD Code 0422 2572265 Mobile Number +91 – 98947 59942 / +91 99410 08018 E-Mail [email protected] Website of the Organization www.psgsonscharities.org 2.1. Name and Address of the Trustee Name Shri. L. Gopalakrishnan Managing Trustee, PSG & SONS’ CHARITIES, Address Post Box No.1609, Avinashi Road, Peelamedu City & Pin Code Coimbatore – 641 004. -
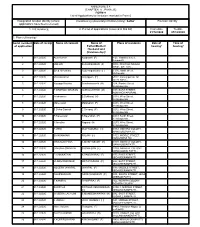
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications For
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): Sattur Revision identity applications have been received) 1. List number@ 2. Period of applications (covered in this list) From date To date 01/12/2020 01/12/2020 3. Place of hearing * Serial number$ Date of receipt Name of claimant Name of Place of residence Date of Time of of application Father/Mother/ hearing* hearing* Husband and (Relationship)# 1 01/12/2020 Arun Kumar Kalidhash (F) 4-20, Vadakku street, Konmpatti, , 2 01/12/2020 MOHAN SUBRAMANIAN (F) 590C, PERIYAR NAGAR WEST, SATTUR, , 3 01/12/2020 Syed Ali fathima Raja mugamathu (F) 2-506, North street, Mettamalai, , 4 01/12/2020 dhanalakshmi kaliyappan (F) 51/22, kamraj puram 1st street, sattur, , 5 01/12/2020 Karpaga Kavitha Sundaramoorthi (H) 2/24, Pambu Street, Vellaiyapuram, , 6 01/12/2020 R DHARANI DHARAN GURULAKSHMI (M) 1/99, EAST STREET, KOMANGIYAPURAM, , 7 01/12/2020 Krishnaveni B.Sokkaraj (H) 2/118, West Street, Vellaiyapuram, , 8 01/12/2020 Mareeswari Malaiyandi (F) 2/246, West Street, Vellaiyapuram, , 9 01/12/2020 S.Selva Ganesh C.Selvaraj (F) 2/232, West Street, Vellaiyapuram, , 10 01/12/2020 R.Kaleeswari K.Rajendran (F) 2/313, North Street, Vellaiyapuram, , 11 01/12/2020 N.Amutha Nagaraj (H) 2/270, West Street, Vellaiyapuram, , 12 01/12/2020 K.VENI M.AYYADURAI (H) 2/112, INDHIRA COLONY, MANJALODAIPATTI, , 13 01/12/2020 SIVASANKARI PALANI (H) 1/122, MIDDLE STREET, KONGANKULAM, , 14 01/12/2020 M.MUKENTHIRA -

Final List of Applicants Who Have Applied for the Post Of
Page 1 FINAL PROVISIONAL LIST OF APPLICANTS WHO HAVE APPLIED FOR THE POST OF TASK FORCE COMMANDER GR-1 (ASST. ENGINEER) Whether Rural/ P.H./ Percenta Sl. Appl Date of Qualifica Max Marks Ex- Name of Candidate Address Father's Name ge of Place of Birth District Caste Category Remarks No. No Birth tion Marks Obtained servicemen/ Marks Lady Candidates 1 516 Nayana A S # LIG 291, 22nd Cross, Kuvempunagar, Hassan- Shive Gowda 14-Sep-89 BE(Civil) 6100 5299 86.87 Aladahally Hassan Vokkaliga 3A Lady -- 573201 2 380 Hemalatha M D D/o Doreswamy M, "Sri Lakshmi" Near Nethaji Doreswamy M 24-Sep-89 BE(Civil) 6100 5214 85.48 Hassan Hassan GM GM Lady -- Public School, Ashok Road, Vidyanagar, Hassan- 573201 3 176 Swathi P Gowda C/o K P Krishne Gowda, Siddeshwara Nilaya, Puttaswamy Gowda 22-Aug-88 BE(Civil) 6800 5789 85.13 Alur Hassan Vokkaliga 3A Rural/ Lady -- Near Holy Children School, Jayanagar 1st Block, Hassan-573201 4 569 Mohamed Hussain S/o Noorjahan, #2, Bunder Road, 1st Cross, Ahamed Bawa 11-Jul-87 BE(Civil) 6675 5573 83.49 Bhatkal Karwar Muslim 2B - -- Bhatkal-581320 5 617 Bharath Kumar P K S/o Kumarappa P, New Police Quarters, Hosalli Kumarappa P 3-Mar-89 BE(Civil) 83.30 Kadur Chickamagalur GM 3B - -- Road, Kadur Tq, Chikkamagalure-577548 6 621 Sushma Mathad #67, "Parvati Nilaya", 4th Main, 7th Cross, Sangayya 12-Jan-90 BE(Civil) 82.90 Chitradurga Chitradurga Lingayath GM Lady -- Gandhinagar, Dharwad-580004 7 513 Sagar S R Kittagalale Village, K Hoskote (P), Alur (T), Ramesh S M 20-Mar-89 BE(Civil) 6800 5623 82.69 Sakelshpur Hassan Vokkaliga 3A Rural -- Hassan (D)-573129.