Touchoncology Touchexpert OPINIONS Acds in Solid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -

Antibody–Drug Conjugates
Published OnlineFirst April 12, 2019; DOI: 10.1158/1078-0432.CCR-19-0272 Review Clinical Cancer Research Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index Steven Coats1, Marna Williams1, Benjamin Kebble1, Rakesh Dixit1, Leo Tseng1, Nai-Shun Yao1, David A. Tice1, and Jean-Charles Soria1,2 Abstract Since the first approval of gemtuzumab ozogamicin nism of activity of the cytotoxic warhead. However, the (Mylotarg; Pfizer; CD33 targeted), two additional antibody– enthusiasm to develop ADCs has not been dampened; drug conjugates (ADC), brentuximab vedotin (Adcetris; Seat- approximately 80 ADCs are in clinical development in tle Genetics, Inc.; CD30 targeted) and inotuzumab ozogami- nearly 600 clinical trials, and 2 to 3 novel ADCs are likely cin (Besponsa; Pfizer; CD22 targeted), have been approved for to be approved within the next few years. While the hematologic cancers and 1 ADC, trastuzumab emtansine promise of a more targeted chemotherapy with less tox- (Kadcyla; Genentech; HER2 targeted), has been approved to icity has not yet been realized with ADCs, improvements treat breast cancer. Despite a clear clinical benefit being dem- in technology combined with a wealth of clinical data are onstrated for all 4 approved ADCs, the toxicity profiles are helping to shape the future development of ADCs. In this comparable with those of standard-of-care chemotherapeu- review, we discuss the clinical and translational strategies tics, with dose-limiting toxicities associated with the mecha- associated with improving the therapeutic index for ADCs. Introduction in antibody, linker, and warhead technologies in significant depth (2, 3, 8, 9). Antibody–drug conjugates (ADC) were initially designed to leverage the exquisite specificity of antibodies to deliver targeted potent chemotherapeutic agents with the intention of improving Overview of ADCs in Clinical Development the therapeutic index (the ratio between the toxic dose and the Four ADCs have been approved over the last 20 years (Fig. -

Anti-HER3 Monoclonal Antibody Patritumab Sensitizes Refractory Non-Small Cell Lung Cancer to the Epidermal Growth Factor Receptor Inhibitor Erlotinib
Oncogene (2016) 35, 878–886 © 2016 Macmillan Publishers Limited All rights reserved 0950-9232/16 www.nature.com/onc ORIGINAL ARTICLE Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib K Yonesaka1, K Hirotani2, H Kawakami1, M Takeda1, H Kaneda1, K Sakai3, I Okamoto4, K Nishio3, PA Jänne5,6,7 and K Nakagawa1 Human epidermal growth factor receptor (HER) 3 is aberrantly overexpressed and correlates with poor prognosis in non-small cell lung cancer (NSCLC). Patritumab is a monoclonal antibody against HER3 that has shown promising results in early-phase clinical trials, but an optimal target population for the drug has yet to be identified. In the present study, we examined whether heregulin, a HER3 ligand that is also overexpressed in a subset of NSCLC, can be used as a biomarker to predict the antitumorigenic efficacy of patritumab and whether the drug can overcome the epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) resistance induced by heregulin. Patritumab sensitivity was associated with heregulin expression, which, when abolished, resulted in the loss of HER3 and AKT activation and growth arrest. Furthermore, heregulin overexpression induced EGFR TKI resistance in NSCLC cells harbouring an activating EGFR mutation, while HER3 and AKT activation was maintained in the presence of erlotinib in heregulin-overexpressing, EGFR-mutant NSCLC cells. Sustained HER3-AKT activation was blocked by combining erlotinib with either anti-HER2 or anti-HER3 antibody. Notably, heregulin was upregulated in tissue samples from an NSCLC patient who had an activating EGFR mutation but was resistant to the TKI gefitinib. -

Genmab and Seattle Genetics Present Data from Tisotumab Vedotin Innovatv 204 Pivotal Trial in Recurrent Or Metastatic Cervical Cancer at ESMO Virtual Congress 2020
Genmab and Seattle Genetics Present Data from Tisotumab Vedotin innovaTV 204 Pivotal Trial in Recurrent or Metastatic Cervical Cancer at ESMO Virtual Congress 2020 Media Release COPENHAGEN, Denmark and BOTHELL, Wash., 21 September 2020 • Data featured in late-breaking proffered paper oral presentation • Biologics license application submission planned to support accelerated approval pathway with the FDA Genmab A/S (Nasdaq: GMAB) and Seattle Genetics, Inc. (Nasdaq: SGEN) today presented data from the innovaTV 204 pivotal phase 2, single-arm clinical trial evaluating tisotumab vedotin as monotherapy in patients with previously treated recurrent and/or metastatic cervical cancer at the European Society for Medical Oncology (ESMO) Virtual Congress 2020. Patients had previously received a doublet chemotherapy and, if eligible, bevacizumab as first-line therapy. Results from the trial showed a 24 percent confirmed objective response rate (ORR) by independent central review with a median duration of response (DOR) of 8.3 months. The most common treatment-related adverse events (greater than or equal to 20 percent) included alopecia, epistaxis (nose bleeds), nausea, conjunctivitis, fatigue and dry eye. Tisotumab vedotin is an investigational antibody-drug conjugate (ADC) directed to tissue factor (TF), which is prevalent on solid tumors including cervical cancer and can promote tumor growth, angiogenesis and metastasis.1 Current therapies for previously treated recurrent and/or metastatic cervical cancer generally result in limited objective response rates of typically less than 15 percent with median overall survival ranging from 6.0 to 9.4 months.1-8 “Following resistance to or progression on first-line standard of care therapy, there are limited treatment options for women with metastatic cervical cancer,” said Robert L. -
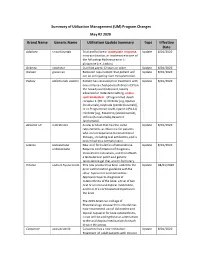
Summary of Utilization Management (UM) Program Changes May #2 2020
Summary of Utilization Management (UM) Program Changes May #2 2020 Brand Name Generic Name Utilization Update Summary Type Effective Date Adakveo crizanlizumab Trial and failure or inadequate response, Update 8/01/2020 contraindication, or intolerance to one of the following: Hydroxyurea or L- glutamine (i.e., Endari) Oxbryta voxelotor Clarified age to 12 years or older Update 8/01/2020 Givlaari givosiran Removed requirement that patient will Update 8/01/2020 not be anticipating liver transplantation. Padcev enfortumab vedotin Patient has received prior treatment with Update 8/01/2020 one immune checkpoint inhibitors (CPI) in the neoadjuvant/adjuvant, locally advanced or metastatic setting, unless contraindicated: i) Programmed death receptor-1 (PD-1) inhibitor [e.g.,Opdivo (nivolumab), Keytruda (pembrolizumab)], or ii) Programmed death-ligand 1 (PD-L1) inhibitor [e.g., Tecentriq (atezolizumab), Imfinzi (durvalumab), Bavencio (avelumab)]. Absorica LD isotretinoin A new product that has the same Update 8/01/2020 requirements as Absorica: for patients who are unresponsive to conventional therapy, including oral antibiotics, and is prescribed by a dermatologist. Jatenzo testosterone New oral formulation of testosterone. Update 8/01/2020 undecanoate Requires confirmation of diagnosis, testosterone lab values, and trial of both a testosterone patch and generic testosterone gel that are on formulary. Triluron sodium hyaluronate This new product has been added to the Update 08/01/2020 prior authorization guideline with the other hyaluronic acid derivatives. Approval requires diagnosis of osteoarthritis of the knee, a trial of two oral or an oral and topical medication, and trial of a corticosteroid injection in the knee. The 2019 American College of Rheumatology Osteoarthritis Guidelines now recommend use of duloxetine and topical capsaicin for knee osteoarthritis, so we will be adding these as alternatives to the oral/topical medications for each drug in this group. -

Antibody–Drug Conjugates: the Last Decade
pharmaceuticals Review Antibody–Drug Conjugates: The Last Decade Nicolas Joubert 1,* , Alain Beck 2 , Charles Dumontet 3,4 and Caroline Denevault-Sabourin 1 1 GICC EA7501, Equipe IMT, Université de Tours, UFR des Sciences Pharmaceutiques, 31 Avenue Monge, 37200 Tours, France; [email protected] 2 Institut de Recherche Pierre Fabre, Centre d’Immunologie Pierre Fabre, 5 Avenue Napoléon III, 74160 Saint Julien en Genevois, France; [email protected] 3 Cancer Research Center of Lyon (CRCL), INSERM, 1052/CNRS 5286/UCBL, 69000 Lyon, France; [email protected] 4 Hospices Civils de Lyon, 69000 Lyon, France * Correspondence: [email protected] Received: 17 August 2020; Accepted: 10 September 2020; Published: 14 September 2020 Abstract: An armed antibody (antibody–drug conjugate or ADC) is a vectorized chemotherapy, which results from the grafting of a cytotoxic agent onto a monoclonal antibody via a judiciously constructed spacer arm. ADCs have made considerable progress in 10 years. While in 2009 only gemtuzumab ozogamicin (Mylotarg®) was used clinically, in 2020, 9 Food and Drug Administration (FDA)-approved ADCs are available, and more than 80 others are in active clinical studies. This review will focus on FDA-approved and late-stage ADCs, their limitations including their toxicity and associated resistance mechanisms, as well as new emerging strategies to address these issues and attempt to widen their therapeutic window. Finally, we will discuss their combination with conventional chemotherapy or checkpoint inhibitors, and their design for applications beyond oncology, to make ADCs the magic bullet that Paul Ehrlich dreamed of. Keywords: antibody–drug conjugate; ADC; bioconjugation; linker; payload; cancer; resistance; combination therapies 1. -

1 Oncologic Drugs Advisory Committee (ODAC)
Oncologic Drugs Advisory Committee (ODAC) Meeting April, 27, 2021 BLA# 761034/Supplement 18 Drug name: TECENTRIQ® (atezolizumab) Applicant: Genentech, Inc. Combined FDA and Applicant ODAC Briefing Document Advisory Committee Briefing Materials: Available For Public Release 1 DISCLAIMER STATEMENT The attached package contains background information prepared by the Applicant and the Food and Drug Administration (FDA) for the panel members of the advisory committee. The package contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought the drug TECENTRIQ® (also known as atezolizumab) to this Advisory Committee in order to gain the Committee’s insights and opinions, and the background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered and all reviews have been finalized. The final determination may be affected by issues not discussed at the advisory committee meeting. 2 Table of Contents Table of Tables ......................................................................................................................... -

FDA Updates Highlighting the Latest Cancer Treatments
FDA Updates Highlighting the Latest Cancer Treatments Orphan Drug Designation of NUV-422 for (mCRC) or squamous cell carcinoma of the head and neck (SCCHN). Malignant Gliomas This approval provides for a biweekly dosage regimen option in The FDA has granted Orphan Drug Designation to NUV-422, a cyclin- addition to the previously approved weekly dosage regimen for the dependent kinase (CDK) 2/4/6 inhibitor, for the treatment of patients approved indications when cetuximab is used as a single agent or in with malignant gliomas. combination with chemotherapy. Patient enrollment and dosing is ongoing in the Phase I/II study The approval was based on population pharmacokinetic (PK) of NUV-422 in adult patients with recurrent or refractory high-grade modeling analyses that compared the predicted exposures of cetux- gliomas, including glioblastoma multiforme. The Phase I dose-esca- imab 500 mg Q2W to observed cetuximab exposures in patients who lation part of the study is designed to evaluate safety and tolerability, received cetuximab 250 mg weekly. The application was also supported as well as determine a recommended Phase II dose based on the toler- by pooled analyses of overall response rates, progression-free survival, ability profile and pharmacokinetic properties of NUV-422. The Phase and overall survival (OS) from published literature in patients with II dose expansion part of the study is expected to initially focus on CRC and SCCHN, and OS analyses using real-world data in patients patients with high-grade gliomas, and it is designed to evaluate overall with mCRC who received either the weekly cetuximab or Q2W regi- response rate, duration of response, and survival. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency. -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

Advanced Development of Erbb Family-Targeted Therapies in Osteosarcoma Treatment
Investigational New Drugs (2019) 37:175–183 https://doi.org/10.1007/s10637-018-0684-8 REVIEW Advanced development of ErbB family-targeted therapies in osteosarcoma treatment Wei Wang1 & Hua-fu Zhao2 & Teng-fei Yao1 & Hao Gong1 Received: 19 August 2018 /Accepted: 16 October 2018 /Published online: 24 October 2018 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Summary Osteosarcoma (OS) is the most common primary aggressive and malignant bone tumor. Newly diagnostic OS patients benefit from the standard therapy including surgical resection plus radiotherapy and neoadjuvant chemotherapy (MAP chemotherapy: high-dose methotrexate, doxorubicin and cisplatin). However, tumor recurrence and metastasis give rise to a sharp decline of the 5-year overall survival rate in OS patients. Little improvement has been made for decades, urging the development of more effective therapeutic approaches. ErbB receptor family including EGFR, HER2, HER3 and HER4, being important to the activation of PI3K/Akt and MAPK signaling pathways, are potential targets for OS treatment. Genetic aberrations (amplification, overexpression, mutation and altered splicing) of ErbB are essential to the growth, apoptosis, motility and metastasis in a variety of cancers. Overexpression of ErbB family is associated with the poor prognosis of cancer patients. A number of monoclonal antibodies or inhibitors specific for ErbB family have entered clinical trials in a range of solid tumors including breast carcinoma, lung carcinoma and sarcoma. Here, we summarized