The Ecological Role of Bivalve Shellfish Aquaculture in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of the Biology and Fisheries of Horse Clams (Tresus Capax and Tresus Nuttallii)
Fisheries and Oceans Pêches at Océans Canada Canad a Canadian Stock Assessment Secretariat Secrétariat canadien pour l'évaluation des stocks Research Document 98/8 8 Document de recherche 98/8 8 Not to be cited without Ne pas citer sans permission of the authors ' autorisation des auteurs ' A Review of the Biology and Fisheries of Horse Clams (Tresus capax and Tresus nuttallii) R. B . Lauzier, C . M. Hand, A. Campbell and S .Heizerz Fisheries and Oceans Canada Pacific Biological Station, Stock Assessment Division, Nanaimo, B.C. V9R 5K6 2 Fisheries and Oceans Canada South Coast Division, N anaimo, B.C. V9T 1K3 ' This series documents the scientific basis for the ' La présente série documente les bases scientifiques evaluation of fisheries resources in Canada . As des évaluations des ressources halieutiques du such, it addresses the issues of the day in the time Canada. Elle traite des problèmes courants selon les frames required and the documents it contains are échéanciers dictés. Les documents qu'elle contient not intended as definitive statements on the subjects ne doivent pas être considérés comme des énoncés addressed but rather as progress reports on ongoing définitifs sur les sujets traités, mais plutôt comme investigations . des rapports d'étape sur les études en cours . Research documents are produced in the official Les documents de recherche sont publiés dans la language in which they are provided to the langue officielle utilisée dans le manuscrit envoyé Secretariat. au secrétariat . ISSN 1480-4883 Ottawa, 199 8 Canada* Abstract A review of the biology and distribution of horse clams (Tresus capax and Tresus nuttallii)and a review of the fisheries of horse clams from British Columbia, Washington and Oregon is presented, based on previous surveys, scientific literature, and technical reports . -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Common Clams, Cockles, Scallops, Oysters Of
CommonHow Clams, Toxic Are Cockles, Alaska's Most Scallops, Common Shellfish Oysters ? of Alaska Concentric Pacific Littleneck Clam rings Protothaca staminea Pacific Razor Clam Distribution: Aleutian Islands to mid-California Alaska Razor Clam Siliqua patula Habitat: Midtidal to subtidal zone, mud to coarse Siliqua alta Distribution: Bristol Bay to southern gravel beaches 1 Distribution: Bering Sea to Cook Inlet California Size: Up to 2 ⁄2" Habitat: Intertidal zone, open coasts in sand Identification: External surface of shell with radiating Habitat: Intertidal zone to 30 feet on open sandy beaches Size: Up to 8" and concentric grooves Horse (Gaper) Clam Size: Up to 6" Identification: Long narrow shell, thin and Tresus capax brittle, olive green to brown color Identification: Long narrow shaped Distribution: Shumagin Islands, Alaska to shell, shell thin and brittle, brown to olive California green color Habitat: Intertidal zone, imbedded deeply Butter Clam Spiny Scallop Size: Up to 8" Saxidomus giganteus Chlamys hastata Identification: Shell large and thick, wide gape Radiating Distribution: Aleutian Islands to mid- Distribution: Gulf of Alaska between shells at posterior end when held grooves California to California together, dark covering on shell surface often or rib Habitat: Intertidal zone to 120 feet depth, on Habitat: Low intertidal area to partially worn off protected gravel, sandy beaches 400 feet depth Blue Mussel 1 Size: Up to 5" Size: Up to 3 ⁄2" Mytilus edulis Identification: Dense shell, external surface Identification: -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
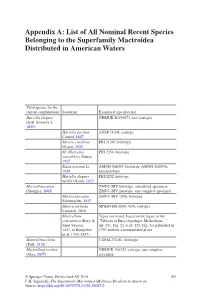
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Recreational Shellfish Harvesting in Pacific County
Dungeness Crab Manila Clam Razor Clam Pacific Oyster Recreational Shellfish Harvesting In Pacific County Cancer magister Littleneck Clam Silliqua patula Cancer magister Clinocardium nuttallii Tapes philippinarum INTRODUCTION out if there are any beach closures in the area. This booklet was prepared through a You must respect the public combined effort of the Pacific and private beaches in County Department of Community Pacific County. Development, Washington State Department of Natural Resources We hope you enjoy this booklet and and Washington State Department find it useful. of Fish and Wildlife. Its purpose is to provide general information regarding the location of public TIDELAND OWNERSHIP beaches, access to these beaches, shellfish available and potential The shores of navigable tidal waters, public health problems associated lying between the line of ordinary with shellfish. high tide and the line of extreme low tide, are considered tidelands. Unfortunately, the authors cannot Many of the tidelands in Willapa Bay guarantee the accuracy of the are privately owned. Every effort has information contained within this been made to verify public tideland booklet due to changes in water locations on these maps; however, quality, land ownership and shellfish we are not responsible for errors. populations. Be sure to adhere to Tideland users should first verify the information contained within this property boundaries. The absence booklet regarding private tideland of posted signs does not ownership, trespassing and access. automatically identify public tidelands. Shellfish may not be taken Remember, before you harvest from private tidelands without the shellfish in Washington State: owner’s permission. You must buy a Washington State shellfish license and HISTORY OF PUBLIC TIDELANDS wear that license while you are harvesting. -

Alaska Final Report
Final Report Chugach Regional Resources Commission Bivalve Enhancement Program Bivalve inventories and native littleneck clam (Protothaca staminea) culture studies Exxon Valdez Oil Spill Trustee Council Project Number 95131 Produced by: Dr. Kenneth M. Brooks Aquatic Environmental Sciences 644 Old Eaglemount Road Port Townsend, Washington 98368 February 2, 2001 Chugach Regional Resources Commission Bivalve Enhancement Program – Bivalve Inventories and native littleneck clam (Protothaca staminea) culture studies Table of contents Page Introduction 1 1.0. Background information 2 1.1. Littleneck clam life history 2 1.1.1. Reproduction 3 1.1.2. Distribution as a function of tidal elevation. 3 1.1.3. Substrate preferences 3 1.1.4. Habitat Suitability Index (HIS) for native littleneck clams. 3 1.2. Marking clams and other bivalves 5 1.3. Aging of bivalves 6 1.4. Length at age for native littleneck clams in Alaska 8 1.5. Bivalve predators 8 1.6. Bivalve culture 9 1.7. Clam culture techniques 10 1.7.1. Predator control 11 1.7.2. Supplemental seeding 11 1.7.3. Substrate modification. 11 1.7.4. Plastic netting 11 1.7.5. Plastic clam bags 12 1.8. Commercial clam harvest management in Alaska 12 1.9. Environmental effects associated with bivalve culture 13 1.10. Background summary 15 1.11. Purpose of this study 15 2.0. Materials, methods and results for the bivalve inventories conducted in 1995 and 1996 at Port Graham, Nanwalek, Tatitlek, Chenega and Ouzinke. 17 2.1. 1995-96 bivalve inventory sampling design. 17 2.2. Clam sample processing. 18 2.3. -

BIOLOGICAL .~ FISHERIES DATA on SURF CLAM, Spisula Solidissima (Dillwyn)
BIOLOGICAL .~ FISHERIES DATA ON SURF CLAM, Spisula solidissima (Dillwyn) FEBRUARY 1980 Biological and Fisheries Data on the Atlantic Surf Clam, Spisula solidissima (Dillwyn) by John W. Ropes Woods Hole Laboratory Northeast Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Administration U.S. Department of Commerce Woods Hole, Massachusetts 02543 Tech. Ser. Rep. No. 24 February 19BO CONTENTS 1. IDENTITY 1.1 Nomencl ature .............................................. 1 1.1.1 Valid Name .......................................... 1 . 1.1.2 Objecti ve Synonymy.................................. 1 1 .2 Taxonomy.. 1 1.2.1 Affinities .......................................... 1 1.2.2 Taxonomic Status .................................... 3 1.2.3 Subspeci es. .. ......... ....... ... .................... 3 1.2.4 Standard Common Names, Vernacular Names ............. 4 1.3 Morphology ................................................. 4 1.3.1 External Morphology (Shell)......................... 4 1 .3.2 Cytomorphology...... 4 1.3.3 Protein Specificity ................................. 4 2. DISTRIBUTION 2.1 Total Area ................................................ 4· 2.2 Differential Distribution.. ..........•..................... 5 2.3 Determinants of Distribution ............................... 9 2.4 Hybridization ............................................. 13 3. BIONOMICS AND LIFE HISTORY 3. 1 Rerroduct i on. .. 13 3 .. 1 Sexuality ........................................... 13 3.1.2 Maturity .....•..................................... -

Venerid Bibliography References for the "Generic Names" Database As Well As Other Selected Works on the Family Veneridae
PEET Bivalvia Venerid Bibliography References for the "Generic Names" database as well as other selected works on the family Veneridae Accorsi Benini, C. (1974). I fossili di Case Soghe - M. Lungo (Colli Berici, Vicenza); II, Lamellibranchi. Memorie Geopaleontologiche dell'Universita de Ferrara 3(1): 61-80. Adachi, K. (1979). Seasonal changes of the protein level in the adductor muscle of the clam, Tapes philippinarum (Adams and Reeve) with reference to the reproductive seasons. Comparative Biochemistry and Physiology 64A(1): 85-89 Adams, A. (1864). On some new genera and species of Mollusca from the seas of China and Japan. Annals and Magazine of Natural History 13(3): 307-310. Adams, C. B. (1845). Specierum novarum conchyliorum, in Jamaica repertorum, synopsis. Pars I. Proceedings of the Boston Society of Natural History 12: 1-10. Ahn, I.-Y., G. Lopez, R. E. Malouf (1993). Effects of the gem clam Gemma gemma on early post-settlement emigration, growth and survival of the hard clam Mercenaria mercenaria. Marine Ecology -- Progress Series 99(1/2): 61-70 (2 Sept.) Ahn, I.-Y., R. E. Malouf, G. Lopez (1993). Enhanced larval settlement of the hard clam Mercenaria mercenaria by the gem clam Gemma gemma. Marine Ecology -- Progress Series 99(1/2): 51-59 Alemany, J. A. (1986). Estudio comparado de la microestructura de la concha y el enrollamiento espiral en V. decussata (L. 1758) y V. rhomboides (Pennant, 1777) (Bivalvia: Veneridae). Bollettino Malacologico 22(5-8): 139-152. Alemany, J. A. (1987). Estudio comparado de la microestructura de la concha y el enrollamiento espiral en Dosinia exoleta (L. -

Gathering Safe Shellfish in Washington Avoiding Paralytic Shellfish Poisoning GATHERING SAFE SHELLFISH in WASHINGTON
LOUISA NISHITANI, KENNETH K. CHEW AND TERI L. KING Gathering Safe Shellfish in Washington Avoiding Paralytic Shellfish Poisoning GATHERING SAFE SHELLFISH IN WASHINGTON This document is published in Fond of shellfish? Washington waters offer a delectable variety part by Grant NA1GRG1044, Project A/FP-7, from the National Oceanic and of clams, oysters, mussels and scallops, readily available to be Atmospheric Administration to Washington Sea Grant, University of Washington. gathered and enjoyed. For seafood lovers, opportunities abound Washington Sea Grant services at many locales and most seasons of the year. “When the tide is and publications are available to all without discrimination. The out, the table is set,” as one beachcomber’s adage maintains. views herein are those of the author and do not necessarily reflect the views of NOAA or any of its sub-agencies. At certain times, however, some shellfish may become unsafe Copyright 2004, University of to eat because they contain a poison harmful to human beings. Washington, Board of Regents Paralytic shellfish poisoning, commonly known as PSP, is a WSG-AS 12-02 danger that you as a shellfish consumer can avoid by being well Revised 06/15 informed and by observing certain basic precautions. This publication provides information about PSP, its symptoms and treatment and its relationship to so-called “red tides.” It also explains what is being done to protect recreational harvesters from this hazard, so that they — and you — can safely enjoy the bounty of Washington seashores. Note: All commercially harvested shellfish must meet rigorous health standards established by the state. At seafood markets and grocery stores in Washington, consumers can purchase nutritious, delicious and safe shellfish that have been certified for sale throughout the year. -

LIBI Appendix A
Lummi Intertidal Baseline Inventory Appendix A: Intertidal Biota Survey Prepared by: Lummi Natural Resources Department (LNR) 2616 Kwina Rd. Bellingham, WA 98226 Contributors: Craig Dolphin LNR Fisheries Shellfish Biologist Michael LeMoine LNR Fisheries Habitat Biologist Jeremy Freimund LNR Water Resources Manager March 2010 A B C D Collecting Intertidal Biota Survey samples during the LIBI. A: Anthony George and Dacia Wiitala. B: Dewey Solomon and Dan Haught. C: Jordan Ballew and Johnathan Robinson D. Delanae Estes and Jessica Urbanec. E. Julie Barber E Executive Summary The Intertidal Biota Survey documented the presence, relative abundance, and preferred habitats of benthic infauna that are present on the Lummi Reservation tidelands. 366 sites were characterized and sampled across the Lummi Reservation tidelands, and the biota present were identified and counted. Approximately 150 taxa were identified in the samples, with some taxonomic labels including more than one species. Overall, polychaete worms in the Family Oweniidae were the most abundant animal taxon present across the Reservation tidelands, followed by caprellid amphipods (Caprella sp.), and then horn shells (Batillaria attramentaria). The most abundant clam species was the recently arrived purple varnish clam (Nuttallia obscurata), which also had the largest total biomass (19.9 million pounds) of any clam species. Butter clam (Saxidomus gigantea), cockle (Clinocardium nuttali), Manila clam (Venerupis phillipinarum), and Pacific littleneck clam (Leukoma staminea) populations had 6.7, 2.7, 2.9, and 2.1 million pounds of biomass, respectively. Horse clam (Tresus sp.) and softshell clam (Mya arenaria) populations were also present but estimates of biomass indicate lower overall biomass for these species (1.6 and 1.2 million pounds, respectively). -

The Spawning Cycle and Juvenile Growth Rate of the Gaper Clam, Tresus Nutalli, of Elkhorn Slough, California
Moss Landing Marine P. 0. !Jox 223 . Moss Calif. 95039 THE SPAWNING CYCLE AND JUVENILE GROWTH RATE OF THE GAPER CLAM, TRESUS NUTALLI, OF ELKHORN SLOUGH, CALIFORNIA A thesis submitted to the faculty of San Francisco State College in partial fulfillment of the requirements for the degree Master of Arts by LAURENCE L. LAURENT San Francisco, California June, 1971 ACKNOWLEDGMENTS I am deeply indebted to several people for their help in bringing this paper into existence. To Dr. James Nybakken of the Moss Landing Marine Laboratories a very special thanks is owed for his guidance, advice, help and concern given throughout the duration of this study. To Dr. Robert Beeman of San Francisco State College go my regard and gratitude for several reasons: for sparking the initial excitement of Marine Biology in undergraduate courses, for his ready accessibility to questions, complaints and the general trials and tribu lations of student life, and, not least of all, for his friendship. To Pat Clark, a fellow graduate student who joined me late in the study, go my thanks for taking on the juvenile sampling and for his much needed help with the statistical end of things; I would also like to express my gratitude to the California Department of Fish and Game, Marine Resources, for their financial support of this study through grant number S-1556. To my wife Sandra I owe much that can't be expressed. On top of all the support, love, encourage- ment and understanding she has given over the five years of our marriage, she is now preparing to give me the iii greatest gift of all this July, 1971--a child.