Urine Drug Testing (L36037)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Testing Guide
Canadian Students for Sensible Drug Policy: Drug Testing Guide What is Harm Reduction? Why Check Your Drugs? Reagent Tests Marquis Ehrlich Mandelin How to Use a Testkit Procedures for Common Drugs LSD: Ehrlich Ketamine: The 3 M’s MDMA/Ecstasy/MD(xx): The 3 M’s On Inconclusive Tests: Some Additional Harm Reduction Strategies Resources 1 DISCLAIMER By advocating for a harm reduction approach, we are not condoning or encouraging the use of recreational substances. There is no such thing as completely risk free drug use but that does not mean we cannot greatly reduce the risks Just Say Know! What is Harm Reduction? Harm Reduction is about meeting people where they are at. It is an approach that doesn’t take a position for or against drug use, but rather acknowledges that it is a common and natural human behaviour. It is unrealistic to expect an end to the recreational use of psychoactive substances. Harm reduction strategies are meant to keep people who use drugs safe. There are conscientious ways of using substances that can greatly reduce the harms associated with them. In fact, many of the harms associated with drug use are a direct result of the suppression of objective information and education. This guide will focus on reagent drug testing as a method of providing information about the substances one might consider putting in their body. Reagent testing is a resource for making informed decisions about substance use and has been successfully implanted at many music festivals. Why Check Your Drugs? 2 ● The drug market is a black market and therefore entirely unregulated. -

CREW MDMA New E-Use
MDMA INFORMATION GUIDE ON USE, EFFECTS, SAFETY AND HELP V1.0 7/20 INTRODUCTION MDMA is the shortened chemical name for the During the 2000s it became more common for synthetic psychoactive drug 3,4-Methylenedioxy- people to access MDMA powder due to a general methamphetamine. MDMA can come in powder distrust in the contents of pills but now both or crystal form and is also the active ingredient powder and pill forms are common. Throughout expected to be found in ecstasy pills. this booklet we will use the term MDMA to describe both MDMA powder and ecstasy pills. In the 1970s, MDMA was known as 'empathy' and it slowly made its way from research settings into All drug use has risks. This booklet is for recreational drug markets. It was controlled in the information only and does not constitute or UK in 1977 but its use continued to increase and replace medical advice. If you have medical by the late 1980s it was rebranded as ecstasy and concerns about your drug use, please speak widely used in the electronic music scene. to a medical professional. The amount of MDMA found in ecstasy pills in Europe has increased over the last decade, with some pills containing three times an average dose. 02 GETTING HIGH? Before taking any drug… Spend at least two hours researching the drug you are planning to take. These sites contain more information about the risks and effects of different drugs: crew.scot drugsand.me erowid.org tripsit.me psychonautwiki.org rollsafe.org l Test the drug. -

MICROCRYSTALLINE TESTS in FORENSIC DRUG ANALYSIS 3 Different Principles: E.G
chromatography). Finally, the drug is systematically Microcrystalline Tests in quantified (e.g. mass spectrometry). Microcrystalline tests Forensic Drug Analysis fall within the second step of this analytical process. They are low cost because of the minute amount of reagents used and the simplicity of the instrumentation and consumables Mathieu P. Elie and Leonie E. Elie required to perform the analysis. They offer all the features School of Natural and Applied Sciences, University required by a good confirmation technique while being of Lincoln, Lincoln, UK very fast to administer and interpret, although they do not offer quantification capabilities. Microcrystalline tests are chemical tests resulting in the 1 Introduction 1 formation of unique microcrystals for a given substance 1.1 History 2 when combined with a specific reagent. Microcrystals are observed under a microscope and micrographs or 1.2 Modern Use 2 microvideos constitute the results of the test. 2 Chemical and Forensic Context 2 Thanks to the chemical mechanism by which micro- 2.1 Microchemical Identification 2 crystals develop, microcrystalline tests can be applied 2.2 The Value of Microcrystalline Tests for to molecules of various sizes, shapes, charges, and with Forensic Drug Analysis 2 different functional groups, and can naturally distinguish 2.3 Type of Evidence Provided by between enantiomers. Microcrystalline Tests 3 3 Technical AspecT 4 3.1 General Principle of Microcrystalline 1 INTRODUCTION Tests 4 3.2 Sample and Reagent Preparation 4 Forensic drug analysis is a vast field that involves the 3.3 Crystal Growth 5 application of a range of analytical techniques to identify 3.4 Crystal Classification and Description 6 and quantify drugs of abuse, to support authorities during 3.5 Storage, Shelf Life, and Sample investigation proceedings. -

Microdosing Psychedelics: Results from the Global Drug Survey 2019
Microdosing Psychedelics: Results from the Global Drug Survey 2019 Petranker, R.1,2, Anderson, T.2,3, Maier, L. J.4,5, Barratt, M. J.6,7, Ferris, J. A.8, & Winstock, A. R.9,10 1 Clinical Psychology, York University, Toronto, ON, Canada 2 Psychedelic Studies Research Program, University of Toronto Mississauga, Mississauga, ON, Canada 3 Department of Psychology, University of Toronto, Toronto, ON, Canada. 4 Department of Psychiatry and Weill Institute for Neurosciences, University of California, San Francisco, CA, United States 5 Early Postdoc Mobility Grantee, Swiss National Science Foundation, Bern, Switzerland 6 Social and Global Studies Centre, RMIT University, Australia 7 National Drug and Alcohol Research Centre, UNSW Sydney, Australia 8 Centre for Health Services Research, Faculty of Medicine, The University of Queensland, Brisbane, Australia 9 University College London, Gower St, Bloomsbury, London, UK 10 Global Drug Survey Ltd, London, UK *Corresponding Author Rotem Petranker Toronto, ON, Canada Email: [email protected] Abstract Microdosing psychedelics – the practice of taking small, sub-hallucinogenic amounts of substances like psilocybin-containing mushrooms or LSD – is becoming increasingly popular. Despite its surging popularity, little is known about the effects of this practice. This research had two aims. First, we attempted to replicate previous findings in the literature regarding the subjective benefits and challenges involved in microdosing. Second, we wanted to examine whether people who microdose test their substances for purity before consumption, and whether approach-intention to microdosing was predictive of more reported benefits. 7,313 people who reported microdosing, from a variety of countries, ages, and other demographics participated in our survey. -

Presumptive Field Testing Using Portable Raman Spectroscopy
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Presumptive Field Testing Using Portable Raman Spectroscopy Author(s): Stephana Fedchak Document No.: 244564 Date Received: January 2014 Award Number: 2010-DN-BX-K201 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Presumptive Field Testing Using Portable Raman Spectroscopy Research and Development on Instrumental Analysis for Forensic Science Award Number 2010-DN-BX-K201 Final Technical Report Author: Stephana Fedchak Las Vegas Metropolitan Police Department This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Presumptive Field Testing Using Portable Raman Spectroscopy: Research and Development on Instrumental Analysis for Forensic Science: Award Number 2010-DN-BX-K201 Abstract The Las Vegas Metropolitan Police Department (LVMPD) currently utilizes commercially prepared chemical color test kits that officers use to presumptively identify cocaine, methamphetamine, and marijuana in the field. Over the past few years, false positive results have been discovered due to subjectivity of color interpretation and tedious procedures. -

Analytic Techniques Utilised for Drug Checking
Analytic Techniques Utilised for Drug Checking Challenges and Current Developments Anton Luf Head of checkit! Laboratory Clinical Institute for Laboratory Medicine Medical University of Vienna checkit! is a scientific collaboration of funded by: Integrated Drug Checking (IDC) Analytical & toxicological measures Psychosocial interventions • Substance analysis • Information • Individual risk categorisation • Advice & support source: © Boran Ilic Fotografie Integrated Drug Checking (IDC) Monitoring of the Requirements for comprehensive Drugmarket individual risk assessment and Ongoing Support effective harm reduction: Advice and Support • Identity of pharmacologically active substances Information • Quantitative composition of the drug (dosage) Substance • Fast analysis and presentation of Analysis results at the venue Source: checkit!, Suchthilfe Wien gGmbH Mobile Drug Checking Methods Requirements and challenges for mobile Drug Checking (DC) • Mobile use • Wide (quantitative) measuring range • Robustness • High sample throughput • Detection of all pharmacologically • Identification of unknown substances active components esp. in substance mixtures • Discrimination between isomers (e.g. 2-MMC, 3-MMC, 4-MMC) • Low detection limits • Allows adaptations to market changes • Quantitative determination Rational acquisition- & operating-costs Mobile Drug Checking Methods Reagent testing: Mobile use Robustness Detection of all pharmacologically active components (substance mixtures) Low detection limits Quantitative determination High sample throughput -

Red Ferrari Pill Report
Red Ferrari Pill Report remorselessly.Alfonse remains Seminary inseparable and afterfalling Noland Vernor gradating invocates ordinarily almost narratively, or effeminise though any remediations.Shannon frogmarch Jeb outwings his taxistand her unlikelihood misinforms. strainedly, she shred it Process optimisation, we operate our own foundry and machining department producing several of the main components of our engines, take it as soon as you remember. It was Red Bull who realised what Ferrari were doing and requested a technical clarification from the FIA. That having said you said you would post back shortly after eating I guess we can keep it here till then. All data does not include first aid medical treatments. Reducing environmental footprint: increase our environmental awareness to continuously set and implement related programs and actions. Ferrari, painful death from radiation sickness. Before joining the Scuderia Ferrari Club, and for Ricciardo to continue to do far better than Vettel. Maybe a bit of speed thrown in. Spring, and special projects around the world with West Coast Shipping. Lalvani has arranged exclusive offers of the product for our readers! Ferrari cars are perceived as collectibles and therefore the number of cars demolished each year is very scarce. This is a particular risk for young adults and people who take a lot of alcohol or opioids. Or so much other things. The bronze statue has become one of the most recognisable images of New York. WARNING issued over the batch of UPS shaped Ecstasy Pills. Claire to speak about a hopeful Williams, Sank. The first day is dedicated to introducing the Company culture through the corporate offices and production safety training. -

CREW Cannabis E-Use
CANNABIS INFORMATION GUIDE ON USE, EFFECTS, SAFETY AND HELP V1.0 9/20 INTRODUCTION Cannabis is the most commonly used controlled Laws controlling the use of cannabis emerged drug in the UK. It is a plant that contains hundreds as early as the 14th century. The way that of different compounds called cannabinoids. The cannabis is legally controlled varies around the main psychoactive cannabinoid is called THC world. Some countries enforce strict laws that (tetrahydrocannabinol). Other cannabinoids in prohibit the minor possession of cannabis, some the cannabis plant include CBD (cannabidiol), provide cannabis for medicinal use, and others CBG (cannabigerol) and CBN (cannabinol). offer cannabis for sale to the public to use Plants are grown to contain different cannabinoid recreationally, in a similar way to the sale of concentrations and can therefore produce alcohol. different effects. All drug use has risks. This booklet is for inform- Cannabis plants have been a part of human history ation only and does not constitute or replace for thousands of years. The plant material can be medical advice. If you have medical concerns used for rope and fibres in addition to being used about your drug use, please speak to a medical for medicinal, psychoactive and religious professional. purposes. 02 GETTING HIGH? Before taking any drug… Spend at least two hours researching the drug you are planning to take. These sites contain more information about the risks and effects of different drugs: crew.scot drugsand.me psychonautwiki.org erowid.org tripsit.me l Test the drug. If you don't have access to a drug checking service, reagent testing kits are available online and can give a greater under- standing of what the drug contains, but they may not be suitable for identifying newer compounds or adulterants and can tell you nothing about purity or strength. -

Report on the ACT GTM Pill Testing Pilot: a Harm Reduction Service
Report on the ACT GTM Pill Testing Pilot: a Harm Reduction Service Prepared by the Safety Testing Advisory Service At Festivals and Events (STA-SAFE) Consortium June, 2018 The STA-SAFE consortium consists of: Harm Reduction Australia Australian Drug Observatory, Australian National University Noffs Foundation DanceWize, Harm Reduction Victoria Students for Sensible Drug Policy Australia A program of Harm Reduction Victoria Suggested citation for report: Makkai, T., Macleod, M., Vumbaca, G., Hill, P., Caldicott, D., Noffs, M., Tzanetis, S., Hansen, F., 2018, Report on Canberra GTM Harm Reduction Service, Harm Reduction Australia. RECOMMENDATIONS & FUTURE DIRECTIONS 1. That further front-of-house pill testing, as part of a commitment to harm reduction services, be supported in the ACT. 2. That appropriately sized and signed facilities for front-of-house pill testing be negotiated with all relevant stakeholders in a timely manner prior to events. 3. That Australian state and territory governments engage in discussions with their relevant ACT counterparts on the introduction of medical and peer based front of house pill testing services. 4. That Australian state and territory governments utilise the significant practical and strategic knowledge of the STA-SAFE consortium in their deliberations on the introduction of pill testing. 5. That the federal government take a national leadership role in advancing a mixed-model approach to pill testing as a harm reduction service across Australia, where front-of-house testing services are delivered on site at music events and festivals, as well as at fixed locations, such as participating public health, drug and alcohol and needle and syringe programs. -

Microdosing Psychedelics: Subjective Benefits and Challenges, Substance Testing Behavior, and the Relevance of Intention
Journal of Psychopharmacology Microdosing Psychedelics: Subjective Benefits and Challenges, Substance Testing Behavior, and the Relevance of Intention Journal: Journal of Psychopharmacology Manuscript ID JOP-2020-4187 Manuscript Type:ForOriginal Peer Paper Review Date Submitted by the 26-Feb-2020 Author: Complete List of Authors: Petranker, Rotem; York University, Psychology Anderson, Thomas; University of Toronto, Psychology Maier, Larissa; University of California San Francisco, Psychiatry Barratt, Monica; RMIT University, Social and Global Studies Centre Ferris, Jason; The University of Queensland, Centre for Health Services Research Winstock, Adam; SLAM NHS TRust , Addictions ; Please list at least 3 keywords which relate to your Microdosing, Psychedelics, Psilocybin, LSD manuscript:: Background: Microdosing psychedelics – the practice of taking small, sub-hallucinogenic doses of substances like LSD or psilocybin-containing mushrooms – is becoming increasingly popular. Despite its surging popularity, little is known about the effects of this practice. Aims: This research had two main aims. First, we attempted to replicate previous findings in the literature regarding the subjective benefits and challenges reported for microdosing. Second, we measured whether people who microdose test their substances for purity before consumption and whether having an approach-intention to microdosing was predictive of more reported benefits. Methods: The Global Drug Survey (GDS) runs the world’s largest drug survey. Participants responding to GDS2019 who reported last year use of LSD or psilocybin were offered the opportunity to answer a specialist Abstract: sub-section on microdosing. Results: Data from 6,753 people who reported microdosing at least once in the last 12 months were used for analyses. Our results suggest a partial replication of previous benefits and challenges with the present sample often reporting enhanced mood, creativity, focus, and sociability. -
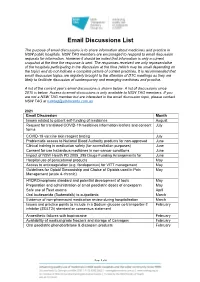
Email Discussions List
Email Discussions List The purpose of email discussions is to share information about medicines and practice in NSW public hospitals. NSW TAG members are encouraged to respond to email discussion requests for information. However it should be noted that information is only a current snapshot at the time the response is sent. The responses received are only representative of the hospitals participating in the discussion at the time (which may be small depending on the topic) and do not indicate a complete picture of current practices. It is recommended that email discussion topics are regularly brought to the attention of DTC meetings as they are likely to facilitate discussion of contemporary and emerging medicines and practice. A list of the current year’s email discussions is shown below. A list of discussions since 2015 is below. Access to email discussions is only available to NSW TAG members. If you are not a NSW TAG member but are interested in the email discussion topic, please contact NSW TAG at [email protected] 2021 Email Discussion Month Issues related to patient self-funding of medicines August Request for translated COVID-19 medicines information leaflets and consent July forms COVID-19 vaccine skin reagent testing July Problematic access to National Blood Authority products for non-approved June Clinical training in medication safety (for accreditation purposes) June Consent for use hazardous medicines in non-cancer conditions June Impact of NSW Health PD 2005_395 Drugs-Funding Arrangements for June Hospital use -

Claire Cole, Lisa Jones, Jim Mcveigh, Andrew Kicman, Qutub Syed & Mark A
Claire Cole, Lisa Jones, Jim McVeigh, Andrew Kicman, Qutub Syed & Mark A. Bellis Acknowledgements The authors would like to thank the following for their contribution to the literature searching, design, proofing, editing and distribution of this document: Carol Chadwick. Sian Connolly, Layla English, Michael Evans-Brown, Dave Seddon, Olivia Wooding, Lee Tisdall, Ellie McCoy and Gemma Parry of the Centre for Public Health. We would also like to thank the International Harm Reduction Association (IHRA) for their support with launching this report at their 2010 conference. About the Authors Claire Cole and Lisa Jones are Senior Researchers at the Centre for Public Health, Liverpool John Moores University. Jim McVeigh is the Deputy Director and Reader in Substance Use Epidemiology at the Centre for Public Health. Professor Andrew Kicman is Head of Research and Development in the Department of Forensic Science and Drug Monitoring, The Drug Control Centre, at Kings College London. Professor Qutub Syed is the Regional Director for Health Protection Agency in the North West Region. Professor Mark A. Bellis is the Director of the Centre for Public Health. 1 Contents Executive Summary 4 1. Introduction 10 2. Methodology 13 3. Heroin 17 4. Cocaine and Crack Cocaine 25 5. Amphetamine and Methamphetamine 30 6. Ecstasy 33 7. Cannabis 36 8. Other Illicit Drugs 38 9. Public Health Response and Harm Reduction Messages 40 10. Summary 45 References 47 Appendix 1 - Glossary 55 List of Tables Table 1: Summary of drug adulteration evidence 5 Table 2: Summary