Modulation of Ryanodine Receptor Ca2+ Channels (Review)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interplay Between Gating and Block of Ligand-Gated Ion Channels
brain sciences Review Interplay between Gating and Block of Ligand-Gated Ion Channels Matthew B. Phillips 1,2, Aparna Nigam 1 and Jon W. Johnson 1,2,* 1 Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA; [email protected] (M.B.P.); [email protected] (A.N.) 2 Center for Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA * Correspondence: [email protected]; Tel.: +1-(412)-624-4295 Received: 27 October 2020; Accepted: 26 November 2020; Published: 1 December 2020 Abstract: Drugs that inhibit ion channel function by binding in the channel and preventing current flow, known as channel blockers, can be used as powerful tools for analysis of channel properties. Channel blockers are used to probe both the sophisticated structure and basic biophysical properties of ion channels. Gating, the mechanism that controls the opening and closing of ion channels, can be profoundly influenced by channel blocking drugs. Channel block and gating are reciprocally connected; gating controls access of channel blockers to their binding sites, and channel-blocking drugs can have profound and diverse effects on the rates of gating transitions and on the stability of channel open and closed states. This review synthesizes knowledge of the inherent intertwining of block and gating of excitatory ligand-gated ion channels, with a focus on the utility of channel blockers as analytic probes of ionotropic glutamate receptor channel function. Keywords: ligand-gated ion channel; channel block; channel gating; nicotinic acetylcholine receptor; ionotropic glutamate receptor; AMPA receptor; kainate receptor; NMDA receptor 1. Introduction Neuronal information processing depends on the distribution and properties of the ion channels found in neuronal membranes. -

Molecular Biology of Neuronal Voltage-Gated Calcium Channels
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 30, No 3, 123-130, September 1998 Molecular biology of neuronal voltage-gated calcium channels Hemin Chin and is capable of directing expression of calcium channel activity in heterologous expression systems. In the central Genetics Research Branch, Division of Basic and Clinical Neuroscience Research, nervous system (CNS), VGCCs are expressed by five National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland, distinct a1 subunit genes (α1A, α1B, α1C, α1D and α1E), U.S.A. which exhibit further variations due to alternative splicing of the primary RNA transcripts. The α1C and, α1D su b u n i t Accepted 3 August 1998 genes encode dihydropyridine (DHP)-sensitive L-type channels, while the three other α1 subunit genes (α1A, α1B and α1E) give rise to DHP-insensitive P/Q-, N- and R-type channels, respectively. The α2 and δ s u b u n i t proteins are produced by proteolytic cleavage of a larger precursor produced by the single α2-δ gene (Table 1). Introduction Three alternatively spliced variants of the α2 subunit are expressed in a tissue-specific manner. Two variants Calcium ions are important intracellular messengers have been isolated from the brain and skeletal muscle mediating a number of neuronal functions including neuro- (Kim et al., 1992; Williams et al., 1992), and a distinct transmitter release, neurosecretion, neuronal excitation, third splice variant which is expressed in glial cells has survival of eurons, and regulation of gene expression. been recently identified (Puro et al., 1996). In addition to The entry of calcium across the plasmamembrane in the gene encoding the skeletal muscle β subunit, three response to membrane depolarization or activation of 1 other β subunit genes (β2, β3 and β4) have been isolated neurotransmitter receptors represents a major pathway thus far. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

Co-Assembly of N-Type Ca and BK Channels Underlies Functional
Research Article 985 Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain David J. Loane*, Pedro A. Lima‡ and Neil V. Marrion§ Department of Pharmacology and MRC Centre for Synaptic Plasticity, University of Bristol, Bristol, BS8 1TD, UK *Present address: Laboratory for the Study of CNS Injury, Department of Neuroscience, Georgetown University Medical Center, Washington, DC 20057, USA ‡Present address: Dep. Fisiologia, Fac. Ciências Médicas, UNL, 1169-056 Lisboa, Portugal §Author for correspondence (e-mail: [email protected]) Accepted 9 January 2007 Journal of Cell Science 120, 985-995 Published by The Company of Biologists 2007 doi:10.1242/jcs.03399 Summary Activation of large conductance Ca2+-activated potassium and reproduced the interaction. Co-expression of (BK) channels hastens action potential repolarisation and CaV2.2/CaV3 subunits with Slo27 channels revealed rapid generates the fast afterhyperpolarisation in hippocampal functional coupling. By contrast, extremely rare examples pyramidal neurons. A rapid coupling of Ca2+ entry with of rapid functional coupling were observed with co- BK channel activation is necessary for this to occur, which expression of CaV1.2/CaV3 and Slo27 channels. Action might result from an identified coupling of Ca2+ entry potential repolarisation in hippocampal pyramidal neurons through N-type Ca2+ channels to BK channel activation. was slowed by the N-type channel blocker -conotoxin This selective coupling was extremely rapid and resistant GVIA, but not by the L-type channel blocker isradipine. to intracellular BAPTA, suggesting that the two channel These data showed that selective functional coupling types are close. -

An Advance About the Genetic Causes of Epilepsy
E3S Web of Conferences 271, 03068 (2021) https://doi.org/10.1051/e3sconf/202127103068 ICEPE 2021 An advance about the genetic causes of epilepsy Yu Sun1, a, *, †, Licheng Lu2, b, *, †, Lanxin Li3, c, *, †, Jingbo Wang4, d, *, † 1The School of Molecular and Cellular Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801-3633, US 2High School Affiliated to Shanghai Jiao Tong University, Shanghai, 200441, China 3Applied Biology program, University of British Columbia, Vancouver, V6r3b1, Canada 4School of Chemical Machinery and Safety, Dalian University of Technology, Dalian, 116023, China †These authors contributed equally. Abstract: Human hereditary epilepsy has been found related to ion channel mutations in voltage-gated channels (Na+, K+, Ca2+, Cl-), ligand gated channels (GABA receptors), and G-protein coupled receptors, such as Mass1. In addition, some transmembrane proteins or receptor genes, including PRRT2 and nAChR, and glucose transporter genes, such as GLUT1 and SLC2A1, are also about the onset of epilepsy. The discovery of these genetic defects has contributed greatly to our understanding of the pathology of epilepsy. This review focuses on introducing and summarizing epilepsy-associated genes and related findings in recent decades, pointing out related mutant genes that need to be further studied in the future. 1 Introduction Epilepsy is a neurological disorder characterized by 2 Malfunction of Ion channel epileptic seizures caused by abnormal brain activity. 1 in Functional variation in voltage or ligand-gated ion 100 (50 million people) people are affected by symptoms channel mutations is a major cause of idiopathic epilepsy, of this disorder worldwide, with men, young children, and especially in rare genetic forms. -

Activation and Modulation of Ligand-Gated Ion Channels
Physiol. Res. 53 (Suppl. 1): S103-S113, 2004 Activation and Modulation of Ligand-Gated Ion Channels J. KRŮŠEK, I. DITTERT, T. HENDRYCH, P. HNÍK, M. HORÁK, M. PETROVIC, M. SEDLÁČEK, K. SUŠÁNKOVÁ, L. SVOBODOVÁ, K. TOUŠOVÁ, E. UJEC, V. VLACHOVÁ, L. VYKLICKÝ, F. VYSKOČIL, L. VYKLICKÝ JR Department of Cellular Neurophysiology, Institute of Physiology, Academy of Sciences of the Czech Republic, Prague, Czech Republic Received January 20, 2004 Accepted March 1, 2004 Summary Ligand-gated ionic channels are integral membrane proteins that enable rapid and selective ion fluxes across biological membranes. In excitable cells, their role is crucial for generation and propagation of electrical signals. This survey describes recent results from studies performed in the Department of Cellular Neurophysiology, Institute of Physiology ASCR, aimed at exploring the conformational dynamics of the acetylcholine, glutamate and vanilloid receptors during their activation, inactivation and desensitization. Distinct families of ion channels were selected to illustrate a rich complexity of the functional states and conformational transitions these proteins undergo. Particular attention is focused on structure-function studies and allosteric modulation of their activity. Comprehension of the fundamental principles of mechanisms involved in the operation of ligand-gated ion channels at the cellular and molecular level is an essential prerequisite for gaining an insight into the pathogenesis of many psychiatric and neurological disorders and for efficient development of novel specifically targeted drugs. Key words Acetylcholine receptor • GABA receptor • glutamate receptor • NMDA receptor • Vanilloid receptor • TRP receptor • Ionic channel Introduction and Ladislav Vyklický Sn. became founders of the laboratory. Their stays in the prominent laboratories The aim of this review is to outline the current abroad (Dept. -
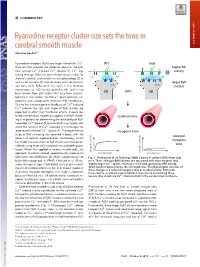
Ryanodine Receptor Cluster Size Sets the Tone in Cerebral Smooth Muscle
COMMENTARY Ryanodine receptor cluster size sets the tone in cerebral smooth muscle COMMENTARY Christian Soellera,1 + Ryanodine receptors (RyRs) are large intracellular Ca2 WT mdx channels that provide the molecular basis of the pro- K+ K+ higher BK + + + cess termed Ca2 -induced Ca2 release (1). Ca2 sig- activity naling through RyRs has been shown to be critical for CaC BK BK skeletal, cardiac, and smooth muscle physiology (2) as well as for neurons (3) and secretory cells like pancre- larger RyR atic beta cells. RyRs were first seen in the electron RyRs RyRs clusters microscope as ∼30-nm-size particles (4), and it had SMCs been known from EM studies that they form clusters, SR SR typically in the narrow “junctional” space between sar- colemma and sarcoplasmic reticulum (SR) membranes. + Due to the inherent positive feedback of Ca2 -induced + Ca2 release, the size and shape of RyR clusters are expected to affect their functional activity. Indeed, de- SMCs SMCs tailed mathematical modeling suggests that RyR cluster- cerebral artery ing is important for determining the excitability of RyR- + mediated Ca2 release (5, 6) and could, in principle, also + affect the amount of Ca2 released in microscopic re- 2+ lease events termed Ca sparks (7). The experimental myogenic tone study of RyR clustering has received a boost with the reduced advent of optical superresolution microscopy, which myogenic has made the assessment of RyR cluster size more acces- sible by using essentially standard immunolabeling pro- tone tocols. When first applied in cardiac muscle cells, this myogenic tone (%) tone myogenic approach revealed a broad, approximately exponential vessel pressure (%) tone myogenic vessel pressure RyR cluster size distribution (8), which had previously not Fig. -

Activation of Trpv1 Channel Contributes to Serotonin-Induced Constriction of Mouse Facial Artery Bolu Zhou University of Vermont
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2017 Activation Of Trpv1 Channel Contributes To Serotonin-Induced Constriction Of Mouse Facial Artery Bolu Zhou University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Pharmacology Commons Recommended Citation Zhou, Bolu, "Activation Of Trpv1 Channel Contributes To Serotonin-Induced Constriction Of Mouse Facial Artery" (2017). Graduate College Dissertations and Theses. 754. https://scholarworks.uvm.edu/graddis/754 This Thesis is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. ACTIVATION OF TRPV1 CHANNEL CONTRIBUTES TO SEROTONIN-INDUCED CONSTRICTION OF MOUSE FACIAL ARTERY A Thesis Presented by Bolu Zhou to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Master of Science Specializing in Pharmacology May, 2017 Defense Date: March 28, 2017 Thesis Examination Committee: George C. Wellman, Ph.D., Advisor Victor May, Ph.D., Chairperson Joseph E. Brayden, Ph.D. Karen M. Lounsbury, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT Tight regulation of cephalic blood circulation is critical under normal physiological conditions, and dysregulation of blood flow to the head occurs in pathophysiological situations such as stroke and migraine headache. The facial artery is an extracranial artery which is one of branches from the external carotid artery territory and its extracranial position indicates its importance in regulating head hemodynamics. -

Transient Receptor Potential Canonical (TRPC) Channels As Modulators of Migration and Invasion
International Journal of Molecular Sciences Review Transient Receptor Potential Canonical (TRPC) Channels as Modulators of Migration and Invasion Muhammad Yasir Asghar 1,2 and Kid Törnquist 1,2,* 1 Minerva Foundation Institute for Medical Research, Biomedicum Helsinki 2U, Tukholmankatu 8, 00290 Helsinki, Finland; yasir.asghar@helsinki.fi 2 Faculty of Science and Engineering, Cell Biology, Åbo Akademi University, Tykistökatu 6A, 20520 Turku, Finland * Correspondence: ktornqvi@abo.fi Received: 11 February 2020; Accepted: 26 February 2020; Published: 3 March 2020 Abstract: Calcium (Ca2+) is perhaps the most versatile signaling molecule in cells. Ca2+ regulates a large number of key events in cells, ranging from gene transcription, motility, and contraction, to energy production and channel gating. To accomplish all these different functions, a multitude of channels, pumps, and transporters are necessary. A group of channels participating in these processes is the transient receptor potential (TRP) family of cation channels. These channels are divided into 29 subfamilies, and are differentially expressed in man, rodents, worms, and flies. One of these subfamilies is the transient receptor potential canonical (TRPC) family of channels. This ion channel family comprises of seven isoforms, labeled TRPC1–7. In man, six functional forms are expressed (TRPC1, TRPC3–7), whereas TRPC2 is a pseudogene; thus, not functionally expressed. In this review, we will describe the importance of the TRPC channels and their interacting molecular partners in the etiology of cancer, particularly in regard to regulating migration and invasion. Keywords: TRPC; ion channels; cancer; thyroid; calcium; migration; invasion; angiogenesis 1. Introduction Increasing evidence during the past decade indicates that different ion channels are expressed in several cancers in humans, and regulate a multitude of cellular processes, including migration, invasion and proliferation [1–3]. -

Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis
cells Review Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis Aurélien Haustrate 1,2, Natalia Prevarskaya 1,2 and V’yacheslav Lehen’kyi 1,2,* 1 Laboratory of Cell Physiology, INSERM U1003, Laboratory of Excellence Ion Channels Science and Therapeutics, Department of Biology, Faculty of Science and Technologies, University of Lille, 59650 Villeneuve d’Ascq, France; [email protected] (A.H.); [email protected] (N.P.) 2 Univ. Lille, Inserm, U1003 – PHYCEL – Physiologie Cellulaire, F-59000 Lille, France * Correspondence: [email protected]; Tel.: +33-320-337-078 Received: 14 October 2019; Accepted: 21 January 2020; Published: 28 January 2020 Abstract: It has been widely established that transient receptor potential vanilloid (TRPV) channels play a crucial role in calcium homeostasis in mammalian cells. Modulation of TRPV channels activity can modify their physiological function leading to some diseases and disorders like neurodegeneration, pain, cancer, skin disorders, etc. It should be noted that, despite TRPV channels importance, our knowledge of the TRPV channels functions in cells is mostly limited to their plasma membrane location. However, some TRPV channels were shown to be expressed in the endoplasmic reticulum where their modulation by activators and/or inhibitors was demonstrated to be crucial for intracellular signaling. In this review, we have intended to summarize the poorly studied roles and functions of these channels in the endoplasmic reticulum. Keywords: TRPV channels; endoplasmic reticulum; calcium signaling 1. Introduction: TRPV Channels Subfamily Overview Functional TRPV channels are tetrameric complexes and can be both homo or hetero-tetrameric. They can be divided into two groups: TRPV1, TRPV2, TRPV3, and TRPV4 which are thermosensitive channels, and TRPV5 and TRPV6 channels as the second group. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title The Effects of Neurosteroids, such as Pregnenolone Sulfate and its receptor, TrpM3 in the Retina. Permalink https://escholarship.org/uc/item/04d8607f Author Webster, Corey Michael Publication Date 2019 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. By Corey Webster A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Molecular and Cell Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Marla Feller, Chair Professor Diana Bautista Professor Daniella Kaufer Professor Stephan Lammel Fall 2019 The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. Copyright 2019 by Corey Webster Abstract The Effects of Neurosteroids, such as Pregnenolone Sulfate, and its receptor, TrpM3 in the Retina. by Corey M. Webster Doctor of Philosophy in Molecular and Cell Biology University of California, Berkeley Professor Marla Feller, Chair Pregnenolone sulfate (PregS) is the precursor to all steroid hormones and is produced in neurons in an activity dependent manner. Studies have shown that PregS production is upregulated during certain critical periods of development, such as in the first year of life in humans, during adolescence, and during pregnancy. Conversely, PregS is decreased during aging, as well as in several neurodevelopmental and neurodegenerative conditions. There are several known targets of PregS, such as a positive allosteric modulator NMDA receptors, sigma1 receptor, and as a negative allosteric modulator of GABA-A receptors. -

Both Gain‐Of‐Function and Loss‐Of‐Function De Novo CACNA1A Mutations Cause Severe Developmental Epileptic Encephalopathies in the Spectrum of Lennox‐Gastaut Syndrome
Received: 30 May 2018 | Revised: 26 July 2019 | Accepted: 29 July 2019 DOI: 10.1111/epi.16316 FULL‐LENGTH ORIGINAL RESEARCH Both gain‐of‐function and loss‐of‐function de novo CACNA1A mutations cause severe developmental epileptic encephalopathies in the spectrum of Lennox‐Gastaut syndrome Xiao Jiang1,2 | Praveen K. Raju1,2 | Nazzareno D'Avanzo3 | Mathieu Lachance1 | Julie Pepin2 | François Dubeau4 | Wendy G. Mitchell5 | Luis E. Bello‐Espinosa6 | Tyler M. Pierson7 | Berge A. Minassian8 | Jean‐Claude Lacaille2 | Elsa Rossignol1,2 1Sainte-Justine University Hospital Center, University of Montréal, Montréal, Canada 2Department of Neurosciences, University of Montréal, Montreal, Canada 3Department of Pharmacology and Physiology, University of Montréal, Montréal, Canada 4Department of Neurosciences, The Montreal Neurological Institute, McGill University, Montréal, Canada 5Neurology Division, Children's Hospital Los Angeles & Department of Neurology, Keck School of Medicine of University of Southern California, Los Angeles, CA, USA 6Department of Clinical Neurosciences, University of Calgary, Alberta, Canada 7Departments of Pediatrics and Neurology, The Board of Governors Regenerative Medicine Institute, Los Angeles, CA, USA 8The Hospital for Sick Children Research Institute, Toronto, Canada Correspondence Elsa Rossignol, Sainte-Justine University Abstract Hospital Center, University of Montréal, Objective: Developmental epileptic encephalopathies (DEEs) are genetically het- Montréal, Canada. erogeneous severe childhood‐onset epilepsies with