Hellenic Plant Protection Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Révision Taxinomique Et Nomenclaturale Des Rhopalocera Et Des Zygaenidae De France Métropolitaine
Direction de la Recherche, de l’Expertise et de la Valorisation Direction Déléguée au Développement Durable, à la Conservation de la Nature et à l’Expertise Service du Patrimoine Naturel Dupont P, Luquet G. Chr., Demerges D., Drouet E. Révision taxinomique et nomenclaturale des Rhopalocera et des Zygaenidae de France métropolitaine. Conséquences sur l’acquisition et la gestion des données d’inventaire. Rapport SPN 2013 - 19 (Septembre 2013) Dupont (Pascal), Demerges (David), Drouet (Eric) et Luquet (Gérard Chr.). 2013. Révision systématique, taxinomique et nomenclaturale des Rhopalocera et des Zygaenidae de France métropolitaine. Conséquences sur l’acquisition et la gestion des données d’inventaire. Rapport MMNHN-SPN 2013 - 19, 201 p. Résumé : Les études de phylogénie moléculaire sur les Lépidoptères Rhopalocères et Zygènes sont de plus en plus nombreuses ces dernières années modifiant la systématique et la taxinomie de ces deux groupes. Une mise à jour complète est réalisée dans ce travail. Un cadre décisionnel a été élaboré pour les niveaux spécifiques et infra-spécifique avec une approche intégrative de la taxinomie. Ce cadre intégre notamment un aspect biogéographique en tenant compte des zones-refuges potentielles pour les espèces au cours du dernier maximum glaciaire. Cette démarche permet d’avoir une approche homogène pour le classement des taxa aux niveaux spécifiques et infra-spécifiques. Les conséquences pour l’acquisition des données dans le cadre d’un inventaire national sont développées. Summary : Studies on molecular phylogenies of Butterflies and Burnets have been increasingly frequent in the recent years, changing the systematics and taxonomy of these two groups. A full update has been performed in this work. -

Some Butterfly Observations in the Karaganda Oblast of Kazakstan (Lepidoptera, Rhopalocera) by Bent Kjeldgaard Larsen Received 3.111.2003
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2003) 34(1/2): 153-165, colour plates Xl-XIVa, Wurzburg, ISSN 0171-0079 Some butterfly observations in the Karaganda Oblast of Kazakstan (Lepidoptera, Rhopalocera) by Bent Kjeldgaard Larsen received 3.111.2003 Abstract: Unlike the Ural Mountains, the Altai, and the Tien Shan, the steppe region of Cen tral Asia has been poorly investigated with respect to butterflies - distribution maps of the re gion's species (1994) show only a handful occurring within a 300 km radius of Karaganda in Central Kazakstan. It is therefore not surprising that approaching 100 additional species were discovered in the Karaganda Oblast during collecting in 1997, 2001 and 2002. During two days of collecting west of the Balkash Lake in May 1997, nine species were identified. On the steppes in the Kazakh Highland, 30 to 130 km south of Karaganda, about 50 butterflies were identified in 2001 and 2002, while in the Karkaralinsk forest, 200 km east of Karaganda, about 70 were encountered. Many of these insects are also to be found in western Europe and almost all of those noted at Karkaralinsk and on the steppes occur in South-Western Siberia. Observations revealed Zegris eupheme to be penetrating the area from the west and Chazara heydenreichi from the south. However, on the western side of Balkash Lake the picture ap peared to change. Many of the butterflies found here in 1997 - Parnassius apollonius, Zegris pyrothoe, Polyommatus miris, Plebeius christophi and Lyela myops - mainly came from the south, these belonging to the semi-desert and steppe fauna of Southern Kazakstan. -

Catalogue No. 121 – Sale, Special Offers and Recent Acquisitions
C. Arden, Bookseller Darren Bloodworth The Nursery, Forest Road, Hay-on-Wye, HR3 5DT, U.K. Tel: +44 (0) 1497-820471 Email: [email protected] Web: www.ardenbooks.co.uk Catalogue No. 121 – Sale, Special Offers and Recent Acquisitions Sale items : Botany 1 - 112 Entomology 113 - 140 Fine, Illustrated & Antiquarian 141 - 151 Gardening 152 - 207 General 208 - 254 Natural History & Zoology 255 - 266 New Naturalist s 267 - 302 Ornithology 303 - 346 Special offers : Botany 347 - 404 and recent Entomology 405 - 440 acquisitions Fine, Illustrated & Antiquarian 441 - 458 Gardening 459 - 512 Natural History & Zoology 513 - 562 New Naturalists 563 - 611 Ornithology 612 - 688 The stock in the Sale part of this catalogue (items 1 to 346) is an attempt to clear the remains of stock from the year’s previous catalogues. Book prices have already been reduced in many cases and further reductions are available to those who wish to take a risk that their chosen books will be available 10 or even 20 days after receiving this catalogue. Books will be dispatched once orders are complete – this may take up to three weeks if you order books at 50% off. How the Sale works First 10 days of sale…….All books available at prices shown in the catalogue After 10 days……………..If books are still available, we reduce their prices by 25% After 20 days……………..If books are still available, we reduce their prices by 50% We have also included over three hundred Special offers and recent acquisitions at the end of the catalogue (items 347 to 688). These Special offers and recent acquisitions are available at the prices indicated and are not part of the Sale terms. -

Redalyc.On the Recent Invasion of the Canary Islands by Two Butterfly
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Wiemers, M.; Acosta-Fernández, B.; Larsen, T. B. On the recent invasion of the Canary Islands by two butterfly species, with the first record of Leptotes pirithous (Linnaeus, 1767) from Gran Canaria, Spain (Lepidoptera: Lycaenidae) SHILAP Revista de Lepidopterología, vol. 41, núm. 161, marzo, 2013, pp. 95-104 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45528755006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 95-104 On the recent invasion o 10/3/13 18:45 Página 95 SHILAP Revta. lepid., 41 (161), marzo 2013: 95-104 CODEN: SRLPEF ISSN: 0300-5267 On the recent invasion of the Canary Islands by two butterfly species, with the first record of Leptotes pirithous (Linnaeus, 1767) from Gran Canaria, Spain (Lepidoptera: Lycaenidae) M. Wiemers, B. Acosta-Fernández & T. B. Larsen Summary Leptotes pirithous is reported from Gran Canaria for the first time, the recent spreading of this species and of Cacyreus marshalli in the Macaronesian Islands is discussed, and distribution maps of both species are presented for the Canary Islands. KEY WORDS: Lepidoptera, Lycaenidae, Leptotes pirithous, Gran Canaria, Spain. Sobre la reciente invasion de dos mariposas en las Islas Canarias con el primer registro de Leptotes pirithous (Linnaeus, 1767) de Gran Canaria, España (Lepidoptera: Lycaenidae) Resumen Se registra por primera vez para Gran Canaria a Leptotes pirithous, se discute la reciente expansión de esta especie y de Cacyreus marshalli en Macaronesia y se dan mapas de distribución de ambas especies presentes en las Islas Canarias. -

Assessing the Indicator Properties of Species Assemblages for Natural Areas Monitoring Author(S): Claire Kremen Source: Ecological Applications, Vol
Assessing the Indicator Properties of Species Assemblages for Natural Areas Monitoring Author(s): Claire Kremen Source: Ecological Applications, Vol. 2, No. 2 (May, 1992), pp. 203-217 Published by: Ecological Society of America Stable URL: http://www.jstor.org/stable/1941776 . Accessed: 07/02/2014 15:50 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Ecological Society of America is collaborating with JSTOR to digitize, preserve and extend access to Ecological Applications. http://www.jstor.org This content downloaded from 128.32.85.74 on Fri, 7 Feb 2014 15:50:07 PM All use subject to JSTOR Terms and Conditions Ecological Applications,2(2), 1992, pp. 203-217 ? 1992 by the Ecological Society of America ASSESSING THE INDICATOR PROPERTIES OF SPECIES ASSEMBLAGES FOR NATURAL AREAS MONITORING' CLAIRE KREMEN Centerfor Conservation Biology, Stanford University, Stanford, California 94305 USA Abstract. The diversityof organismsand complexityof ecosystemsprevent thorough inventoryand monitoringof protectedareas, yet sound databases are needed to manage ecosystems for long-termpersistence. One strategyis thereforeto focus monitoringon indicatororganisms, but guidelinesare lackingfor selecting appropriate species or groups. This paper presentsa simple protocolbased on ordinationtechniques for establishing the indicatorproperties of a group of organismsand forselecting an indicatorspecies subset formore intensivemonitoring. -

Beitrag Zur Lepidopterenfauna Der Insel Mauritius 111-115 Atalanta 42 (1-4): 111-115, Würzburg (2011), ISSN 0171-0079
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Atalanta Jahr/Year: 2011 Band/Volume: 42 Autor(en)/Author(s): Freina Josef J. De Artikel/Article: Beitrag zur Lepidopterenfauna der Insel Mauritius 111-115 Atalanta 42 (1-4): 111-115, Würzburg (2011), ISSN 0171-0079 Beitrag zur Lepidopterenfauna der Insel Mauritius (Lepidoptera, Rhopalocera, Heterocera) von JOSEF J. DE FREINA eingegangen am 6.VI.2011 Zusammenfassung: Die Arbeit liefert eine kommentierte Auflistung der im Februar 2003 vom Verfasser auf Mauritius nachgewie- senen Lepidoptera-Arten, ergänzt durch bekannte und neue Daten über deren Biologien. Der Autor konnte Arten wie Danaus ple- xippus (LINNAEUS , 1758), Chilades pandava (HORSFIE L D , [1829]) und Leptomyrina phidias (FABRICIUS, 1793) beobachten, die, erst seit zwei Jahrzehnten als neu für die Inselfauna gemeldet, inzwischen auf der gesamten Insel heimisch zu sein scheinen. Ein Nachweis für Cacyreus darius MABI ll E , 1877, die seit rund 40 Jahren nicht mehr festgestellt wurde, belegt, daß die Art nicht wie angenommen ausgestorben ist. Schließlich wird die Lepidopterenliste von Mauritius durch den Erstnachweis einer bisher unbekannten Art der Gattung Hyblaea FABRICIUS, 1793 (Hyblaeidae) ergänzt. Abstract: A survey of Lepidoptera, collected by the author on Mauritius in Februar 2003, is listed with some comments. The present paper also reviews current knowledge of Mauritian Lepidoptera bionomic details. The author observed some Mauritian species like Danaus plexippus (LINNAEUS , 1758), Chilades pandava (HORSFIE L D , [1829]), and Leptomyrina phidias (FABRICIUS, 1793), which are recently reported since the last twenty years and which seem now well-established throughout the island. The recent proof of Cacyreus darius MABI ll E , 1877, not seen or taken during the last fourty years and assumed to be extinct, indicates that this spe- cies is still present in the island. -

The Status and Distribution of Mediterranean Butterflies
About IUCN IUCN is a membership Union composed of both government and civil society organisations. It harnesses the experience, resources and reach of its 1,300 Member organisations and the input of some 15,000 experts. IUCN is the global authority on the status of the natural world and the measures needed to safeguard it. www.iucn.org https://twitter.com/IUCN/ IUCN – The Species Survival Commission The Species Survival Commission (SSC) is the largest of IUCN’s six volunteer commissions with a global membership of more than 10,000 experts. SSC advises IUCN and its members on the wide range of technical and scientific aspects of species conservation and is dedicated to securing a future for biodiversity. SSC has significant input into the international agreements dealing with biodiversity conservation. http://www.iucn.org/theme/species/about/species-survival-commission-ssc IUCN – Global Species Programme The IUCN Species Programme supports the activities of the IUCN Species Survival Commission and individual Specialist Groups, as well as implementing global species conservation initiatives. It is an integral part of the IUCN Secretariat and is managed from IUCN’s international headquarters in Gland, Switzerland. The Species Programme includes a number of technical units covering Species Trade and Use, the IUCN Red List Unit, Freshwater Biodiversity Unit (all located in Cambridge, UK), the Global Biodiversity Assessment Initiative (located in Washington DC, USA), and the Marine Biodiversity Unit (located in Norfolk, Virginia, USA). www.iucn.org/species IUCN – Centre for Mediterranean Cooperation The Centre was opened in October 2001 with the core support of the Spanish Ministry of Agriculture, Fisheries and Environment, the regional Government of Junta de Andalucía and the Spanish Agency for International Development Cooperation (AECID). -
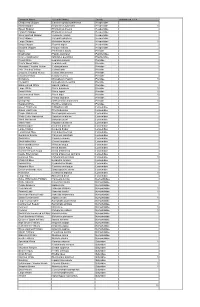
Withymead Butterfly and Dragonfly Survey 2018
Common Name Scientific Name Family Withymead 2018 Chequered Skipper Carterocephalus palaemon Hesperiidae Small Skipper Thymelicus sylvestris Hesperiidae 1 Essex Skipper Thymelicus lineola Hesperiidae Lulworth Skipper Thymelicus acteon Hesperiidae Silver-spotted Skipper Hesperia comma Hesperiidae Fiery Skipper Hylephilla phyleus Hesperiidae Large Skipper Ochlodes faunus Hesperiidae 1 Dingy Skipper Erynnis tages Hesperiidae Grizzled Skipper Pyrgus malvae Hesperiidae Apollo Parnassius apollo Pieridae Swallowtail Papilio machaon Papilionidae Scarce Swallowtail Iphiclides podalirius Papilionidae Wood White Leptidea sinapis Pieridae Réal's Wood White Leptidea reali Pieridae Moorland Clouded Yellow Colias palaeno Pieridae Pale Clouded Yellow Colias hyale Pieridae Berger's Clouded Yellow Colias alfacariensis Pieridae Clouded Yellow Colias croceus Pieridae Brimstone Gonepteryx rhamni Pieridae 1 Cleopatra Gonepteryx cleopatra Pieridae Black-veined White Aporia crataegi Pieridae Large White Pieris brassicae Pieridae 1 Small White Pieris rapae Pieridae 1 Green-veined White Pieris napi Pieridae 1 Bath White Pontia daplidice Pieridae Orange-tip Anthocharis cardamines Pieridae 1 Dappled White Euchloe simplonia Pieridae Green Hairstreak Callophrys rubi Lycaenidae Brown Hairstreak Thecla betulae Lycaenidae Purple Hairstreak Neozephyrus quercus Lycaenidae White Letter Hairstreak Satyrium w-album Lycaenidae Black Hairstreak Satyrium pruni Lycaenidae Slate Flash Rapala schistacea Lycaenidae Small Copper Lycaena phlaeas Lycaenidae Large Copper Lycaena dispar -

The Entomologist's Record and Journal of Variation
. JVASV^iX ^ N^ {/) lSNrNVIN0SHilWS*^S3ldVaan^LIBRARIES SMITHSONIAN INSTITUTION Ni <n - M ^^ <n 5 CO Z ^ ^ 2 ^—^ _j 2 -I RIES SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVdan U r- ^ ^ 2 CD 4 A'^iitfwN r: > — w ? _ ISNI NVINOSHilWS SBiyVdan LIBRARIES'SMITHSONIAN INSTITUTION f^ <rt .... CO 2 2 2 s;- W to 2 C/J • 2 CO *^ 2 RIES SMITHSONIAN_INSTITUTlON NOIiniliSNI_NVINOSHilWS S3liiVyan_L; iiSNi"^NViNOSHiiNS S3iyvaan libraries smithsonian'^institution i^ 33 . z I/' ^ ^ (^ RIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS S3lbVHan Li CO — -- — "> — IISNI NVINOSHimS S3IMVHan LIBRARIES SMITHSONIAN INSTITUTION N' 2 -J 2 _j 2 RIES SMITHSONIAN INSTITUTION NOIifllliSNI NVINOSHIIWS SSIMVyail L! MOTITI IT I f\t _NviN0SHiiws'^S3iMvaan libraries'^smithsonian^institution NOlin z \ '^ ^—s^ 5 <^ ^ ^ ^ '^ - /^w\ ^ /^^\ - ^^ ^ /^rf^\ - /^ o ^^^ — x.ii:i2Ji^ o ??'^ — \ii Z ^^^^^""-^ o ^^^^^ -» 2 _J Z -J , ; SMITHSONIAN INSTITUTION NOIXniliSNI NVINOSHillMS $3 I M VH 8 !!_ LI BR = C/> ± O) ^. ? CO I NVINOSHimS S3iaVHan libraries SMITHSONIAN INSTITUTION NOIlf CO ..-. CO 2 Z z . o .3 :/.^ C/)o Z u. ^^^ i to Z CO • z to * z > SMITHS0NIAN_1NSTITUTI0N NOIiniliSNI_NVINOSHimS S3 I d ViJ 8 n_LI B R UJ i"'NViNOSHiiws S3ibvyan libraries smithsonian"^institution Noiir r~ > z r- Z r- 2: . CO . ^ ^ ^ ^ ; SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHillNS SSiyVMail LI BR CO . •» Z r, <^ 2 z 5 ^^4ii?^^ ^' X^W o ^"^- x life ^<ji; o ^'f;0: i >^ _NVIN0SHiIlMs'^S3iyVdan^LIBRARIEs'^SMITHS0NlAN INSTITUTION NOlif Z \ ^'^ ^-rr-^ 5 CO n CO CO o z > SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHimS S3 I ^Vd 8 11 LI BR >" _ . z 3 ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. SOKOLOFF fre s Assistant Editors J.A. -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Example Insect Natural History Data
Example Insect Natural History Data These data were assembled by participants of a workshop held at the University of Florida from May 30 to June 1 of 2018. The data cover all five major insect orders (Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera) and represent most of the various kinds of natural history information found on insect specimen labels. The data also include representative natural history information from literature sources and online databases. For more information about how these data were assembled and why, see Stucky et al. (2019) __________. Except for works in the public domain, data use licenses are as specified by the original data owners. Coleoptera Example 1 Taxonomy: Coleoptera: Buprestidae: Acmaeodera sp. Record type: database Life stage(s): adult Source: iNaturalist Record URL: https://www.inaturalist.org/observations/12840335 Comments and relevant content: "Feeding on wildflowers in an open meadow in the midlands of South Carolina." Example 2 Taxonomy: Coleoptera: Cerambycidae Record type: literature Source: Paro et al. (2011) Relevant text: "Table 1. Association between girdled and available host-plants (listed alphabetically) and Onciderini beetles in Serra do Japi from 2002 to 2006." The table gives the percentages of each plant species that were girdled along with associated beetle species. Example 3 Taxonomy: Coleoptera: Cerambycidae: Rhaesus serricollis Record type: literature Source: Sama et al. (2010) Relevant text: "Host plants: Polyphagous on deciduous trees like Platanus (Platanaceae), Ficus -

Biogeography and Systematics of Aricia Butterflies (Lepidoptera
Molecular Phylogenetics and Evolution 66 (2013) 369–379 Contents lists available at SciVerse ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Biogeography and systematics of Aricia butterflies (Lepidoptera, Lycaenidae) ⇑ Claudia P. Sañudo-Restrepo a,b, Vlad Dinca˘ a,c, Gerard Talavera a,d, Roger Vila a, a Institut de Biologia Evolutiva (CSIC-Universitat Pompeu Fabra), Passeig Marítim de la Barceloneta 37, 08003 Barcelona, Spain b Facultat de Biologia, Universitat de Barcelona, Avinguda Diagonal 645, 08028 Barcelona, Spain c Department of Zoology, Stockholm University, 106 91 Stockholm, Sweden d Departament de Genètica i Microbiologia, Universitat Autònoma de Barcelona, Bellaterra, 08193 Barcelona, Spain article info abstract Article history: Butterflies of the Aricia species group represent a paradigm of unresolved taxonomy, both at the genus Received 11 November 2011 and species levels. We studied phylogenetic relationships, biogeography, and systematics based on Revised 10 September 2012 genetic – nuclear and mitochondrial – and morphometric – external (wings) and internal (genitalia) – Accepted 10 October 2012 data. We show that Aricia is a monophyletic genus comprising the taxa Pseudoaricia, Ultraaricia and Available online 23 October 2012 Umpria, which are here considered junior synonyms of Aricia. The taxa allous, inhonora, issekutzi, mand- zhuriana, myrmecias and transalaica, which have often been raised to species rank, are shown to probably Keywords: represent subspecies or synonyms. We show that montensis is likely a good species that is sister to all A. Biogeography artaxerxes populations across the Palearctic region. The species A. anteros and A. morronensis are shown to Hybrids Lepidoptera display deep intraspecific divergences and they may harbor cryptic species.