Contra Costa Goldfields)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Butte Co. Meadowfoam
U.S. Fish and Wildlife Service, and the U.S. Air Force, respectively. All of the Fort Ord occurrences are on land within the Habitat Management Plan Habitat Reserve lands and will be conserved and managed in perpetuity (W. Collins in litt. 2005; U.S. Army Corps of Engineers 1997). The population at Travis Air Force Base, including over 20 acres of adjacent restored vernal pools, is protected as a special ecological preserve, with protective measures and appropriate management for the species provided in the Travis Air Force Base Land Management Plan. Seasonal managed cattle grazing has been returned to two conservation sites supporting Lasthenia conjugens: 1) the Warm Springs Seasonal Wetland Unit of the Don Edwards San Francisco Bay National Wildlife Refuge in Alameda County, and 2) the State Route 4 Preserve managed by the Muir Heritage Land Trust in Contra Costa County. The L. conjugens population at the Warm Springs Unit has declined during the last 10 years due to many factors including competition by nonnative plant species. During this time period, grazing, which occurred intermittently at the Warm Springs Unit since the 1800s, has been excluded by the Refuge until a management plan could be developed. The decline in the L. conjugens population at the Warm Springs Unit cannot be attributed to a single factor, but most likely results from the complex interaction of several variables including current and historical land uses, the abiotic environment, and annual climatic variation. The increasing dominance of nonnative grasses, however, coincides with the suspension of livestock grazing, suggesting that the lack of a disturbance regime may be a primary factor in the degradation of habitat for L. -

Loch Lomond Button-Celery)
Natural Diversity Data Base 2003). The Sacramento National Wildlife Refuge populations have been monitored annually since 1992 (J. Silveira in litt. 2000). One additional occurrence of C. hooveri in Merced County is on private land (the Bert Crane Ranch) that is protected from development by a conservation easement (J. Silveira in litt. 2000). We funded a status survey for Chamaesyce hooveri and other vernal pool plants in 1986 and 1987 (Stone et al. 1988), resulting in 10 new occurrences. We and the California Department of Fish and Game jointly funded an ecological study of the Vina Plains Preserve pools, which was conducted by faculty from California State University, Chico (Alexander and Schlising 1997). Independent surveys conducted by Joseph Silveira led to discovery of the Merced and Glenn county occurrences (J. Silveira in litt. 2000). Private landowners also have contributed to conservation of this species. One pool in Tehama County was fenced by the property owner in the late 1980s, to exclude livestock (Stone et al. 1988). 3. ERYNGIUM CONSTANCEI (LOCH LOMOND BUTTON-CELERY) a. Description and Taxonomy Taxonomy.—Loch Lomond button-celery, specifically known as Eryngium constancei (Sheikh 1983), is a member of the carrot family (Apiaceae). This species was only recently described and therefore has no history of name changes. The common name was derived from the type locality, Loch Lomond, which is in Lake County (Sheikh 1983). Other common names for this species are Loch Lomond coyote-thistle (Skinner and Pavlik 1994) and Constance’s coyote-thistle (Smith et al. 1980). Description and Identification.—Certain features are common to species of the genus Eryngium. -

An Investigation of the Reproductive Ecology and Seed Bank
California Department of Fish & Game U.S. Fish and Wildlife Service: Endangered Species Act (Section-6) Grant-in-Aid Program FINAL PROJECT REPORT E-2-P-35 An Investigation of the Reproductive Ecology and Seed Bank Dynamics of Burke’s Goldfields (Lasthenia burkei), Sonoma Sunshine (Blennosperma bakeri), and Sebastopol Meadowfoam (Limnanthes vinculans) in Natural and Constructed Vernal Pools Christina M. Sloop1, 2, Kandis Gilmore1, Hattie Brown3, Nathan E. Rank1 1Department of Biology, Sonoma State University, Rohnert Park, CA 2San Francisco Bay Joint Venture, Fairfax, CA 3Laguna de Santa Rosa Foundation, Santa Rosa, CA Prepared for Cherilyn Burton ([email protected]) California Department of Fish and Game, Habitat Conservation Division 1416 Ninth Street, Room 1280, Sacramento, CA 95814 March 1, 2012 1 1. Location of work: Santa Rosa Plain, Sonoma County, California 2. Background: Burke’s goldfield (Lasthenia burkei), a small, slender annual herb in the sunflower family (Asteraceae), is known only from southern portions of Lake and Mendocino counties and from northeastern Sonoma County. Historically, 39 populations were known from the Santa Rosa Plain, two sites in Lake County, and one site in Mendocino County. The occurrence in Mendocino County is most likely extirpated. From north to south on the Santa Rosa Plain, the species ranges from north of the community of Windsor to east of the city of Sebastopol. The long-term viability of many populations of Burke’s goldfields is particularly problematic due to population decline. There are currently 20 known extant populations, a subset of which were inoculated into pools at constructed sites to mitigate the loss of natural populations in the context of development. -

Recovery Plan for the Santa Rosa Plain
U.S. Fish & Wildlife Service Recovery Plan for the Santa Rosa Plain Blennosperma bakeri (Sonoma sunshine) Lasthenia burkei (Burke’s goldfields) Limnanthes vinculans (Sebastopol meadowfoam) California tiger salamander Sonoma County Distinct Population Segment (Ambystoma californiense) Lasthenia burkei Blennosperma bakeri Limnanthes vinculans Jo-Ann Ordano J. E. (Jed) and Bonnie McClellan Jo-Ann Ordano © 2004 California Academy of Sciences © 1999 California Academy of Sciences © 2005 California Academy of Sciences Sonoma County California Tiger Salamander Gerald Corsi and Buff Corsi © 1999 California Academy of Sciences Disclaimer Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. We, the U.S. Fish and Wildlife Service, publish recovery plans, sometimes preparing them with the assistance of recovery teams, contractors, state agencies, Tribal agencies, and other affected and interested parties. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Costs indicated for action implementation and time of recovery are estimates and subject to change. Recovery plans do not obligate other parties to undertake specific actions, and may not represent the views or the official positions of any individuals or agencies involved in recovery plan formulation, other than the Service. Recovery plans represent our official position only after they have been signed by the Director or Regional Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery actions. LITERATURE CITATION SHOULD READ AS FOLLOWS: U.S. -

Vernal Pool Landscape As Illustrated in the Butte Area Natural Resources Conservation Service Soil Survey ANDREW E
CONTENTS Introduction ..................................................................................................................................... 1 Plants .................................................................................................................................................. 3 Science and Vernal Pool Conservation: Research Questions, Methodologies and Applications Based on a Case Study of Pogogyne abramsii in San Diego County, California ELLEN T. BAUDER ..................................................................................................................... 5 The Population and Genetic Status of the Endangered Vernal Pool Annual Limnanthes floccosa Howell ssp. californica Arroyo: Implications for Species Recovery CHRISTINA M. SLOOP .............................................................................................................. 25 The Ecology, Evolution, and Diversification of the Vernal Pool Niche in Lasthenia (Madieae, Asteraceae) NANCY C. EMERY, LORENA TORRES-MARTINEZ, ELISABETH FORRESTEL, BRUCE G. BALDWIN AND DAVID D. ACKERLY......................................................................................... 39 A Comparison of Pollination Interactions in Natural and Created Vernal Pools in the Santa Rosa Plain, Sonoma County, California JOAN M. LEONG....................................................................................................................... 59 Animals ........................................................................................................................................... -

4.4 BIOLOGICAL RESOURCES 4.4.1 Regulatory Setting
Ascent Environmental Administrative Draft – For Internal Review and Deliberation Biological Resources 4.4 BIOLOGICAL RESOURCES This section describes the potential effects of the project on biological resources. This section also addresses biological resources known or with potential to occur in the project vicinity, including common vegetation and habitat types, sensitive plant communities, and special-status plant and animal species. The analysis includes a description of the existing environmental conditions, the methods used for assessment, the potential direct and indirect impacts of project implementation not included in the 2005 Subsequent Environmental Impact Report (SEIR) for the Hay Road Landfill Project (Solano County 2005), and mitigation measures recommended to address impacts determined to be significant or potentially significant. The data and documents reviewed in preparation of this analysis included: 2005 SEIR for the Hay Road Landfill Project (Solano County 2005); Burrowing Owl Habitat Assessment (ESA 2016a); California Tiger Salamander Habitat Assessment (ESA 2016b); Branchiopod Survey Report (ESA 2016c); Special-status Plant Survey Report (ESA 2016d); Organics Transload Facility Habitat Assessment (ESA 2017a); Hydro Flow Analysis (ESA 2017b); Contra Costa Goldfields Survey Report (ESA 2017c); Delta Green Ground Beetle Survey Report and Supplemental Habitat Assessment Report (Entomological Consulting Services, Ltd. 2016, 2018); reconnaissance-level survey of the project site conducted on August 7, 2017; records search and GIS query of the California Natural Diversity Database (CNDDB) within 5 miles of the project site (2018); California Native Plant Society (CNPS), Rare Plant Program database search of the Allendale, Dixon, Saxon, Elmira, Dozier, Liberty Island, Denverton, Birds Landing, and Rio Vista U.S. Geological Service 7.5-minute quadrangles (CNPS 2018); eBird online database of bird observations (eBird 2018); and aerial photographs of the project site and surrounding area. -

Pollinators in Peril: a Systematic Status Review of North American
POLLINATORS in Peril A systematic status review of North American and Hawaiian native bees Kelsey Kopec & Lori Ann Burd • Center for Biological Diversity • February 2017 Executive Summary hile the decline of European honeybees in the United States and beyond has been well publicized in recent years, the more than 4,000 species of native bees in North W America and Hawaii have been much less documented. Although these native bees are not as well known as honeybees, they play a vital role in functioning ecosystems and also provide more than $3 billion dollars in fruit-pollination services each year just in the United States. For this first-of-its-kind analysis, the Center for Biological Diversity conducted a systematic review of the status of all 4,337 North American and Hawaiian native bees. Our key findings: • Among native bee species with sufficient data to assess (1,437), more than half (749) are declining. • Nearly 1 in 4 (347 native bee species) is imperiled and at increasing risk of extinction. • For many of the bee species lacking sufficient population data, it’s likely they are also declining or at risk of extinction. Additional research is urgently needed to protect them. • A primary driver of these declines is agricultural intensification, which includes habitat destruction and pesticide use. Other major threats are climate change and urbanization. These troubling findings come as a growing body of research has revealed that more than 40 percent of insect pollinators globally are highly threatened, including many of the native bees critical to unprompted crop and wildflower pollination across the United States. -

The Biological Resources Section Provides Background Information
4.5 BIOLOGICAL RESOURCES The Biological resources section provides background information on sensitive biological resources within Napa County, the regulations and programs that provide for their protection, and an assessment of the potential impacts to biological resources of implementing the Napa County General Plan Update. This section is based upon information presented in the Biological Resources Chapter of the Napa County Baseline Data Report (Napa County, BDR 2005). Additional information on the topics presented herein can be found in these documents. Both documents are incorporated into this section by reference. This section addresses biological resources other than fisheries which are separately addressed in Section 4.6. 4.5.1 SETTING REGIONAL SETTING The Napa County is located in the Coast Ranges Geomorphic Province. This province is bounded on the west by the Pacific Ocean and on the east by the Great Valley geomorphic province. A dominant characteristic of the Coast Ranges Province is the general northwest- southeast orientation of its valleys and ridgelines. In Napa County, located in the eastern, central section of the province, this trend consists of a series of long, linear, major and lesser valleys, separated by steep, rugged ridge and hill systems of moderate relief that have been deeply incised by their drainage systems. The County is located within the California Floristic Province, the portion of the state west of the Sierra Crest that is known to be particularly rich in endemic plant species (Hickman 1993, Stein et al. 2000). LOCAL SETTING The County’s highest topographic feature is Mount St. Helena, which is located in the northwest corner of the County and whose peak elevation is 4,343 feet. -

MITIGATED NEGATIVE DECLARATION for the Tesla Road
Draft INITIAL STUDY/ MITIGATED NEGATIVE DECLARATION for the Tesla Road Winery ALAMEDA COUNTY CALIFORNIA Prepared for: Alameda County CDA Planning Division Prepared by: Denise Duffy & Associates Contact: Denise Duffy 947 Cass St. Suite 5 Monterey, California 93940 July 31, 2015 This page left intentionally blank. MITIGATED NEGATIVE DECLARATION Project: Tesla Road Winery Lead Agency: Alameda County PROJECT DESCRIPTION This Mitigated Negative Declaration (MND), supported by the attached Initial Study (IS), evaluates the environmental effects of a proposed multi‐use wine facility at the northeast corner of the Greenville Road and Tesla Road intersection outside of Livermore, within unincorporated Alameda County, California. The applicant, RAO Company, is proposing the construction of a new 19,944 square foot (sq. ft.) building on the the property. The building’s primary function would be to provide space for wine tasting, tours, and special events, and administrative offices for employees. The building would provide a dedicated space to process wine, serve customers, and hold events. Alameda County is the lead agency for this project and has prepared this MND. FINDINGS An IS has been prepared to assess the projects potential effects on the environment and the significance of those effects. Based on the Initial Study, it has been determined that the proposed project would not have any significant effects on the environment once mitigation measures are implemented. This conclusion is supported by the following findings: 1. The proposed project would have no impact related to greenhouse gas emissions, aesthetics, agricultural resources, hazards/hazardous materials, land use/planning mineral resources, population/housing, public services, and recreation. -
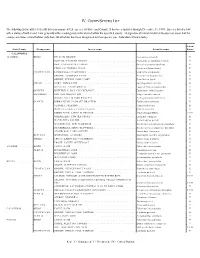
Iv. County/Species List
IV. COUNTY/SPECIES LIST The following list identifies federally listed or proposed U.S. species by State and County. It has been updated through December 31, 1999. Species listed below with a status of both E and T are generally either endangered or threatened within the specified county. Designation of critical habitat (CH) does not mean that the county constitutes critical habitat, only that critical habitat has been designated for that species (see Addendum A Instructions). Action/ State/County Group name Inverse name Scientific name Status CALIFORNIA ALAMEDA ......... BIRDS ....... PELICAN, BROWN ........................................ Pelicanus occidentalis E PLOVER, WESTERN SNOWY ................................ Charadrius alexandrinus nivosus T RAIL, CALIFORNIA CLAPPER ............................... Rallus longirostris obsoletus E TERN, CALIFORNIA LEAST ................................. Sterna antillarum browni E CRUSTACEAN . LINDERIELLA, CALIFORNIA ................................ Linderiella occidentalis E SHRIMP, LONGHORN FAIRY ................................ Branchinecta longiantenna E SHRIMP, VERNAL POOL FAIRY .............................. Branchinecta lynchi T FISHES ...... GOBY, TIDEWATER ...................................... Eucyclogobius newberryi E SPLITTAIL, SACRAMENTO ................................. Pogonichthys macrolepidotus T INSECTS ..... BUTTERFLY, BAY CHECKERSPOT ........................... Euphydryas editha bayensis T MAMMALS ... FOX, SAN JOAQUIN KIT .................................... Vulpes macrotis -

Common Wildflowers of the Coast to Crest Trail
COMMON WILDFLOWERS OF THE COAST TO CREST AND CHINA GULCH TRAILS Mokelumne Watershed & Recreation Division EBMUD March 2015 Compiled using these references: Philip Munz, California Spring Wildflowers Peterson Field Guide, Pacific State Wildflowers Wildflower Walks and Roads of the Sierra Gold Country The Outdoor World of the Sacramento Region Weeds of the West Philip Munz, A California Flora & Supplement Originally compiled by Ranger/Naturalist II Steve Diers Updated by Ranger/Naturalist II Vanessa Stevens Color Scientific Name Common Name Blooms Habitat Notes, Location Blue Cynoglossum grande Hounds Tongue, May-Jul Shady woods MCCT at Gunsight Pass Grand Hounds Blue Eriodictyon californicum YerbaT Santa May-Jul Dry rocky slopes and ridges Turkey Hill Campground Blue Nemophila menziesii Baby Blue-eyes Mar-May Moist fields, slopes MCCT @ Log Boom Blue Lupinus bethamii Bentham's Lupine, Mar-Jun Raodbanks, slopes Sony Creek Road, east of the Spider Lupine Beauna Vista Road Intersection Blue Lupinus bicolor Miniature Lupine Mar-Jun Slopes, gravel Common Blue Lupinus nanus Sky Lupine, Douglas's Apr-May Slopes, grassy Lupine Blue Sisyrinchium bellum Blue-eyed Grass Feb-Jul Grassland Blue Salvia sonomensis Sonoma sage, May-Jun Slopes, chaparral McAffee Gulch Creeping sage Blue Trichostema lanceolatum Wooly Blue Curls, Jun-Oct Waste places Vinegar Weeds Blue Penstemon azureus Azure Pensteman May-Jul Slopes, dry Gunsight Pass Blue Veronica peregrina Speedwell April-Aug Grassland Mokelumne Office ssp. xalapensis Blue Gilia capitata Capitate Gilia, -

Thirteen Plant Taxa from the Northern Channel Islands Recovery Plan
Thirteen Plant Taxa from the Northern Channel Islands Recovery Plan I2 cm Illustration of island barberry (Berberis pinnata ssp. insularis). Copyright by the Regents of the University of California, reproduced with permission of the Jepson Herbarium, University of California. THIRTEEN PLANT TAXA FROM THE NORTHERN CHANNEL ISLANDS RECOVERY PLAN Region 1 U.S. Fish and Wildlife Service Portland, Oregon Approved: Operations Office, Wildlife Service Date: I PRIMARY AUTHOR The Recovery Plan for Thirteen Plant Taxa from the Northern Channel Islands was prepared by: Tim Thomas U.S. Fish and Wildlife Service 1 DISCLAIMER Recovery plans delineate reasonable actions which are believed to be required to recover and/or protect listed species. We (the U.S. Fish and Wildlife Service) publish recovery plans, sometimes preparing them with the assistance of recovery teams, contractors, State agencies, and others. Objectives of the recovery plan will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Costs indicated for task implementation and/or time for achievement ofrecovery are only estimates and are subject to change. Recovery plans do not necessarily represent the views, official positions, or approval ofany individuals or agencies involved in the plan formulation other than our own. They represent our official position only after they have been signed by the Director, Regional Director, or CalifornialNevada Operations Office Manager as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. Literature citation should read: U.S.