Disinfection of Three Wading Boot Surfaces Infested with New Zealand Mudsnails Kelly A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

”Shoes”: a Componential Analysis of Meaning
Vol. 15 No.1 – April 2015 A Look at the World through a Word ”Shoes”: A Componential Analysis of Meaning Miftahush Shalihah [email protected]. English Language Studies, Sanata Dharma University Abstract Meanings are related to language functions. To comprehend how the meanings of a word are various, conducting componential analysis is necessary to do. A word can share similar features to their synonymous words. To reach the previous goal, componential analysis enables us to find out how words are used in their contexts and what features those words are made up. “Shoes” is a word which has many synonyms as this kind of outfit has developed in terms of its shape, which is obviously seen. From the observation done in this research, there are 26 kinds of shoes with 36 distinctive features. The types of shoes found are boots, brogues, cleats, clogs, espadrilles, flip-flops, galoshes, heels, kamiks, loafers, Mary Janes, moccasins, mules, oxfords, pumps, rollerblades, sandals, skates, slides, sling-backs, slippers, sneakers, swim fins, valenki, waders and wedge. The distinctive features of the word “shoes” are based on the heels, heels shape, gender, the types of the toes, the occasions to wear the footwear, the place to wear the footwear, the material, the accessories of the footwear, the model of the back of the shoes and the cut of the shoes. Keywords: shoes, meanings, features Introduction analyzed and described through its semantics components which help to define differential There are many different ways to deal lexical relations, grammatical and syntactic with the problem of meaning. It is because processes. -

Wading for a Bite Fishing from Shore Can Create a Dilemma
Wading for a Bite Fishing from shore can create a dilemma. You may not be able to cast far enough to reach some fish. One trick is wading closer. Chest waders or hip boots will help. Waders and hip boots come in two types— “stocking foot” or “boot foot.” “Boot foot” means the boot is part of the wader or hip boot. “Stocking foot” means a neoprene sock is sewn onto the wader or hip boot. A boot is laced up over top (just like putting shoes over your socks). Both work well, but stocking foot types are usually more comfortable. Waders and hip boots are made of different materials. 48 Pennsylvania Angler & Boater • March/April 2014 PFBC Facebook: PaFishandBoat www.fishandboat.com Rubber – Waders or boots made from rubber are inexpensive and durable. They are good for walking through brush, but they offer no thermal protection from cold water. If they are folded during storage, older rubber boots can leak near creases. Neoprene – Neoprene waders or boots are durable. They are great for wading in cold weather, because neoprene traps body heat but can be less comfortable to wear. Breathable fabric – Breathable fabric allows air and body moisture to pass through but blocks water. It is comfortable for all day wear but won’t trap heat like neoprene. Waders and hip boots also have different types of soles and choosing the right one can be an important decision. Felt – Felt is great on rocky bottom streams. However, walking on muddy bottom streams or muddy banks can be slippery with felt soles. -
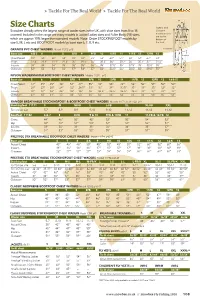
108 Size Chart.Indd
» Tackle For The Real World » Tackle For The Real World Size Charts Inseam and Chest Snowbee already offers the largest range of wader sizes in the UK, with shoe sizes from 5 to 15 Outseam measurements covered. Included in the range are many models in Junior/Ladies sizes and Fuller Body (FB) sizes, are to the which are approx 15% larger than standard models. Note: Order STOCKINGFOOT models by bottom of size S, M, L etc and BOOTFOOT models by boot size 6, 7, 8, 9 etc. the heel. Hip GRANITE PVC CHEST WADERS Model 11072 p48 Outseam Boot size 4 & 5 6 7 8 8FB 9 9FB 10 10FB 11 11FB 12 12FB 13 Inseam Chest/Waist 48" 50" 50" 53" 59" 53" 59" 53" 60" 54" 60" 54" 62" 54" Thigh 27.6" 29.1" 29.1" 29.5" 35" 29.5" 35" 30.3" 36" 30.7" 36" 30.7" 37" 31.5" Inseam 34" 34" 34" 35" 36" 35" 36" 35" 37.5" 35" 37.5" 35" 38.5" 35" Outseam 52" 53" 53" 54" 57" 54" 57" 54" 58.5" 54" 58.5" 55" 59.5" 55" NYLON WADERMASTER BOOTFOOT CHEST WADERS Models 11291 p45 Boot size 5 6 7 8 8FB 9 9FB 10 10FB 11 11FB 12 12FB 13 14&15 Chest/Waist 44” 45” 45” 46” 54” 47” 55” 48” 55” 49” 56” 50” 57” 50” 50” Thigh 26” 27” 28” 29” 32” 29.5” 33” 30” 34” 30.5” 35” 31” 35” 32” 32” Inseam 32” 32” 34” 36” 36” 36” 36” 36.5” 36.5” 36.5” 36.5” 37” 37” 37” 37” Outseam 49” 50” 51” 53” 54” 53” 54” 54” 55” 54” 55” 55” 56” 55” 55” RANGER BREATHABLE STOCKINGFOOT & BOOTFOOT CHEST WADERS Models 11171 and 11182 p44 Stockingfoot 11171 S M M-FB L L-FB XL XL-FB XXL To fi t shoe size 5-7 8-9 8-9 9-10 9-10 11-12 11-12 11-12 Bootfoot 11182 6 & 7 8 8-FB 9 & 10 9FB & 10FB 11 11-FB & 12-FB 12 Chest 44” 46” 50” -

Temperature-Sensing Waders for Environmental Sciences
Geosci. Instrum. Method. Data Syst., 5, 45–51, 2016 www.geosci-instrum-method-data-syst.net/5/45/2016/ doi:10.5194/gi-5-45-2016 © Author(s) 2016. CC Attribution 3.0 License. Proof of concept: temperature-sensing waders for environmental sciences Rolf Hut1, Scott Tyler2, and Tim van Emmerik1 1Water Resources Section, Faculty of Civil Engineering and Geosciences, Delft University of Technology, Delft, the Netherlands 2Dept. of Geological Sciences and Engineering, University of Nevada, Reno, USA Correspondence to: Rolf Hut ([email protected]) Received: 8 November 2015 – Published in Geosci. Instrum. Method. Data Syst. Discuss.: 11 December 2015 Revised: 6 February 2016 – Accepted: 10 February 2016 – Published: 29 February 2016 Abstract. A prototype temperature-sensing pair of waders Briggs et al., 2011; Mwakanyamale et al., 2013). Stream dis- is introduced and tested. The water temperature at the charge, water-level fluctuations, transport of contaminants, streambed is interesting both for scientists studying the hy- and heat exchange all depend on the interaction within the porheic zone and for, e.g., fishers spotting good fishing lo- hyporheic zone (Anderson, 2005; Boano et al., 2012). In- cations. A temperature sensor incorporated into waders worn teractions in the hyporheic zone between groundwater and by members of the public can give scientists an additional surface water is often complex. Because their quantity and source of information on stream-water–groundwater interac- quality can significantly affect each other, understanding the tion. A pair of waders was equipped with a thermistor and principles of the processes in the hyporheic zone is necessary calibrated in the lab. -

Let's Go Fishing Program Manual 1
LET'S GO FISHING PROGRAM MANUAL l 1 COMPOSITE IMAGE © EMPIPE / DREAMSTIME.COM / EMPIPE © IMAGE COMPOSITE Acknowledgments: Special Thank you! Written by: Kyle Glencross and Andrew Schafermeyer Review and Editing: Nicola Whitley, Jay Martin, Becky Johnson, Jennifer Baldi, and Laura Ryder Layout and Design: Victor Young A special thank you to the following for providing us with some content and many images for this publication: Larry Pape, Going Fishing, Nebraska Game and Parks, Aquatic Education Program To all of the volunteers Daniel Griffith, Fishing in the Schools Manual, Oklahoma Department of Wildlife Conservation who make this program Mike Beauchene, Let's Go Fishing!, Connecticut Aquatic Resources Education possible. Additional images provided by Dreamstime.com and iStock.com The N.H. Fish and Game Department receives Federal Assistance from the U.S. Fish and Wildlife Service, and thus Published by prohibits discrimination on the basis of race, color, national origin, disability, age and sex, pursuant to Title VI of New Hampshire Fish and Game Department the Civil Rights Act of 1964, Section 504 of the Rehabilitation Act of 1973, Title II of the Americans with Disabilities Aquatic Resources Education Program Act of 1990, Title IX of the Education Amendments of 1972, and the Age Discrimination Act of 11 Hazen Drive, Concord, NH 03301 1975. If you believe you have been discriminated against in any program, activity or service, ©2020 NH Fish and Game Dept. please contact or write the U.S. Fish & Wildlife Service, Division of Wildlife and Sport Fish Website: fishnh.com Restoration, 4001 N. Fairfax Drive, Mail Stop: WSFR – 4020, Arlington, Virginia 22203, Attention: E-mail: [email protected] Civil Rights Coordinator for Public Programs. -

Clean Your Gear!
Clean Your Gear! Preventing the Spread of Aquatic Invasive Species www.paseagrant.org in Pennsylvania Tips for Anglers Recreational fishing provides both economic and social benefits to Pennsylvania. It is important that anglers take action to remove any “hitchhikers” from their equipment, boots, nets, clothing, and other gear that touched the water while fishing so that AIS are not spread to new locations. Aquatic invasive species (AIS) are non-native plants, animals, or pathogens that cause harm to the environment, the economy, and human or animal health. Preventing the spread of AIS is important because once introduced, these species disrupt ecosystems, reduce biodiversity, and cost communities huge amounts of time, money, resources, and lost revenue. AIS pose a significant threat to recreational and commercial fishing. Native fish populations, including both prey and game fish, are negatively impacted by AIS because they compete for food, reduce spawning habitat, feed on young of year, and impact water quality. Some species can become a serious nuisance to anglers by fouling fishing lines and stealing bait. Aquatic invasive plants also impact fishing because they clog aquatic habitats, limit access to good fishing spots, and make navigation nearly impossible. Because anglers often travel great distances to fish at different lakes Photo courtesy of Rob Carr, Associated Press. and streams, unwanted species can be unintentionally transported and Robert Cousins of Arlington, Va., scrubs his introduced. Hitchhikers can attach themselves to equipment, clothing, boots, and boats felt soled waders at a wader wash station and move from lake to lake. Therefore it is before fishing for trout. -

June 7, 2019 the Honorable Robert
June 7, 2019 The Honorable Robert Lighthizer United States Trade Representative 600 17th Street Northwest Washington, D.C. 20508 Re: Hearing on Section 301 Proposed Actions on China’s Acts, Policies, and Practices Related to Technology Transfer, Intellectual Property, and Innovation (Docket No. USTR-2019-0004) Dear Ambassador Lighthizer: The sportfishing industry requests that sportfishing equipment, including products under heading 9507 of the Harmonized Tariff Schedule of the United States and any other products subject to the federal excise tax on fishing equipment, be excluded from the Section 301 Tariff List. The tariff would not be practicable, and it would cause disproportionate economic harm to most of our industry. The American Sportfishing Association (ASA) represents the sportfishing industry and the entire recreational fishing community. This audience encompasses all the fishing tackle manufacturers and related business associated with recreational fishing, including the almost 50 million Americans that enjoy fishing each year. Since 1933, our association has represented not only the companies that make the rods, reels, lines and lures that anglers need to catch fish, but also the tackle boxes and fishing nets, waders and bobbers, sunglasses and boat shoes, marine electronics, boats and motors. As an industry we understand the position of President Trump regarding the current trade relations with China and we support realigning our trade agreements to correct the unfair trade practices, including the treatment of intellectual property. However, we are deeply concerned about the impacts of these proposed tariffs on all the manufacturers who attempt to provide a service to what the public calls its favorite pastime – fishing. -

Lesson: Water's Living Things: Seining and Water Quality Testing
Arlington Echo/4th Grade Environmental Literacy/Quarter 4 Lessons/Seining and Water Quality DRAFT 3/2017 update Lesson: Water’s Living Things: Seining and Water Quality Testing *Arlington Echo works to continuously improve our lessons. This lesson may be modified over the course of the school year.* Environmental Literacy Question: How have humans affected the Chesapeake Bay and its watershed? Topic/Essential Question: What human activity’s cause erosion? How can we prevent erosion? Unit/Lesson Sequence: One of two lessons in the “Water’s Living Things” 4th grade module based at Arlington Echo Outdoor Education Center. Content Standards: Environmental Literacy 4.A.1.b. Explain and demonstrate food webs for a particular environment. 5.A.1.Analyze the effects of human activities on earth’s natural processes. 8.F.1.b. Identify actions that can be taken as individuals and those that require the involvement of other people, organizations and government. Science 3.F.1.a. Identify and describe the interactions of organisms present in a habitat. 6.B.1. Recognize and describe that people in Maryland depend on, change, and are affected by the environment. 3.A.1.b. Classify a variety of animals and plants according to their observable features and provide reasons for placing them into different groups. Common Core Standards for English Language Arts Standards- Speaking and Listening-4th Grade Comprehension and Collaboration CCSS.ELA-Literacy.SL.4.1 Engage effectively in a range of collaborative discussions (one- on-one, in groups, and teacher-led) with diverse partners on grade 4 topics and texts, building on others’ ideas and expressing their own clearly. -

Next Meeting Sept. 4, 7:00 PM
Wichita, Kansas www.flatlandflyfishers.org Volume 13 Issue 9 Presidents Line September, 2008 Fall is a very busy time for our INSIDE THIS ISSUE club, with our Banquet, Women’s Program, and Trout Art of Building season. It is also the time of A Bamboo Rod year for elections, and Page 2 November is our election ___________________ meeting where you will have the Notes From opportunity to elect or reelect all new officers. I have asked A New Member Kendal Rush to chair an election Page 3 committee in order to facilitate Stuart with a nice Striper ____________________ the election process. The earlier High Sierra we start this process the more time we will have to organize a list of candidates. Fly Fishing New officers are healthy for any organization, bringing in new ideas and Page 4,5 direction so the club does not get stagnated. Elections give equal opportunity for all members to be involved with the decisions and direction. I would urge ____________________ all members to fulfill a position for at least one year. This allows you an Slough Creek Work Day opportunity to see what goes on behind the scenes and gives you a voice in Basic Fly Fishing planning all of the events. These positions are not just for those who have Page 5 been in the club for a long time; they are for any member new or old. The only ____________________ requirements are a desire to see the club prosper and grow. If you would like Fly Fishing Western to be involved then you need to let Kendal know so we can put you on the ballot. -

Vol. 35, No. 2, Spring, 2009
The American Fly Fisher Journal of the American Museum of Fly Fishing SPRING 2009 VOLUME 35 NUMBER 2 Profiles in Fly Fishing THE AMERICAN MUSEUM OF FLY FISHING Preserving the Heritage of Fly Fishing en All FRIENDS OF THE MUSEUM don Gor Alan Airth (’08) Robert Brucker (’08) C. Austin Buck (’08) Larry Cohen (’08) S MANY OF YOU are aware, it’s the Martha. To learn more about this legend Donald Christ (’08) nature of publications for staff to be from one who knew him, turn to page 10. David DiPiero (’08) Aworking on an issue’s words and Kay Brodney, tournament caster, is the Domenic DiPiero (’08) images—like the ones you hold in your subject of this issue’s “Gallery” piece (page John Dreyer Sr. (’08) hands right now—months in advance of 17). Brodney, once a trustee of the museum, Fredrik Eaton (’08) said issue’s in-house arrival. Here we are in donated two early fiberglass fly rods made Michael Geitz (’08) deep-freeze January putting together the by the Reelon Rod Co. Nathan George, in Jon Gibson (’08) last of the words you will read in the Spring “Casting for Action, Not Attention: Kay Thomas Gravina (’08) 2009 issue. By the time you open its pages, Brodney’s Fiberglass Rods,” highlights this James Houghton (’08) even we in the Northeast will be donning our early-1980s acquisition and gives us some John Kaiser (’08) waders and hitting the local trout streams, Brodney background as well. Fred Kambeitz (’08) which today seems like a distant dream. -

2007 Get It Introduction 3 Introduction to Place an Order, Request a Catalog Or Jackson Hole Is Home to Some Big Fish
2007 Get it Introduction 3 Introduction To place an order, request a catalog or Jackson Hole is home to some big fish. A wild and native stock of Spotted Cutthroat is for further information on our products: Wading + FOOTWEAR 4-5 the initial draw but, upon closer inspection, it is clear few locales can claim such diversity of 1. SEE CLOUDVEIL GOODS IN PERSON Wading + WADERS 6-7 Visit one of our fine fishing specialty trout fisheries in such a concentrated area. Whether wading or floating, selecting dry flies, retailers. A complete dealer listing can be Wading + VESTS 8-9 streamers or nymphs, diehards are easily hooked. In this local subculture, South Fork salmon found at the centerfold of this catalog. 10-15 2. CALL CLOUDVEIL Outerwear + HARDSHELL fly hatches earn legendary status, seasonal channeling knowledge of the Maze is a badge CUSTOMER SERVICE Outerwear + FLEECE 16-17 of honor, and Opening Day on Flat Creek is treated as a local holiday. Toll free: 877.255.8345 Monday through Friday 8am-6pm Mountain Outerwear + SOFTSHELL 18-21 Standard Time In this beautiful valley alone, our options for landing one are abundant. During prime Mountain Lifestyle + ACTIVE 26-35 3. SHOP CLOUDVEIL ON THE WEB season, we float Snake River sections–either Pacific to Deadman’s Bar or Wilson to South www.cloudveilfishing.com and Mountain Lifestyle + TOWNWEAR 36-37 www.cloudveil.com Park–and likely find friends lurking at both put-in and take-out. For solitude we head to Base Layer 38-41 stealth holes on the Buffalo Fork or Gros Ventre that are always worth the extra effort. -

Bamboo Rod Making Classes Faqs
Bamboo rod making classes FAQs Q. Do we actually finish a complete bamboo rod during the 6-day class period? A. Yes, you leave the class with a 100% complete and ready to fish, hand crafted bamboo fly rod. Q. I have no experience in woodworking. Will I be able to do this? A. Yes, we teach more bamboo rod making students than anyone in the world, and no one has ever failed to complete a rod they can be proud of. Q. What size and length rod will I make in the class? A. Because you have your own individual workbench and planing form, you may build any size, weight and length 2-piece rod that you desire. We have tapers 0-12 wt. In fact, our saltwater tapers (8-12 wt) are almost as popular as our fresh water tapers. Q. What is included with my tuition? • six days of instruction at the workshop of Bill Oyster • you choose length, line wt. and taper • you customize all cosmetic options from reel seat, to thread wraps, grip shape, and cane tone • all tools, components, hardware, and materials provided • take home a COMPLETE AND READY TO FISH bamboo rod you made yourself! • rod bag and tube with label included • NO EXPERIENCE NECESSARY Q. Is Bill Oyster your instructor? A. Yes, every Oyster Bamboo Rod Building Class is instructed by Bill Oyster. Inside the Oyster workshop Q. Where is your shop located? A. We are located at 494 East Main Street in historic downtown Blue Ridge, GA 30513. Click here to see our location by map.