ACIP Meeting Agenda August 13, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Federal Register/Vol. 85, No. 157/Thursday, August 13, 2020
49398 Federal Register / Vol. 85, No. 157 / Thursday, August 13, 2020 / Notices OFFICE OF PERSONNEL comment period. No comments were OFFICE OF PERSONNEL MANAGEMENT received for this collection. The purpose MANAGEMENT of this notice is to allow an additional Submission for Review: 3206–0162; 30 days for public comments. The Office Excepted Service Report of Medical Examination of of Management and Budget is Person Electing Survivor Benefits, particularly interested in comments AGENCY: Office of Personnel OPM 1530 that: Management (OPM). 1. Evaluate whether the proposed AGENCY: Office of Personnel collection of information is necessary ACTION: Notice. Management. for the proper performance of the ACTION: 30-Day notice and request for functions of the agency, including SUMMARY: This notice identifies comments. whether the information will have Schedule A, B, and C appointing practical utility; SUMMARY: The Retirement Services, authorities applicable to a single agency Office of Personnel Management (OPM) 2. Evaluate the accuracy of the that were established or revoked from offers the general public and other agency’s estimate of the burden of the January 1, 2020 to January 31, 2020. proposed collection of information, Federal agencies the opportunity to FOR FURTHER INFORMATION CONTACT: Julia comment on a revised information including the validity of the methodology and assumptions used; Alford, Senior Executive Resources collection request, OPM 1530, Report of Services, Senior Executive Services and Medical Examination of Person Electing 3. Enhance the quality, utility, and Performance Management, Employee Survivor Benefits. clarity of the information to be collected; and Services, 202–606–2246. DATES: Comments are encouraged and 4. Minimize the burden of the SUPPLEMENTARY INFORMATION: In will be accepted until September 14, collection of information on those who 2020. -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -
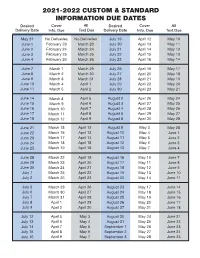
2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
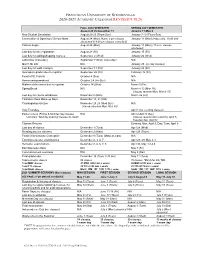
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

2021 Subdivision Calendar
Planning Department Development Review – Major Subdivision Applications First Plat Review 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Deadline for Staff Planning Board BOC Meeting Planning Department Review Deadline Postponement Meeting Date* Date** December 18, 2020 December 31 January 13 January 22 February 2 March 15 January 15 January 29 February 10 February 19 March 2 April 19 February 19 March 5 March 17 March 26 April 6 May 17 March 19 April 2 April 14 April 23 May 4 June 21 April 16 April 30 May 12 May 21 June 1 July 19 May 21 June 4 June 16 June 25 July 6 August 16 June 18 July 2 July 14 July 23 August 3 September 20 July 23 August 6 August 18 August 27 September 7 October 18 August 20 September 3 September 15 September 24 October 5 November 15 September 17 October 1 October 13 October 22 November 2 December 20 October 22 November 5 November 17 November 26 December 7 ▪ January 18, 2022 November 19 December 3 December 15 ▪ December 22 ▪ January 4, ▪ February 21, 2022 2022 ▪ ▪ ▪ December 17 December 31, January 12, ▪ January 21, ▪ February 1, March 21, 2021 2022 2022 2022 2022 * Planning Board Has Two (2) Meetings to Make Recommendation ** Board of Commissioners Has Up to Four (4) Meetings to Make Decision ▪ 2021 Dates are Tentative – Meeting Dates Will Be Set in December 2021 With Adoption of 2022 Meeting Calendars Construction/Final Plat Review – Administrative* 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Planning Review Deadline Department For TRC December 23, 2020 January 6 January 13 January -
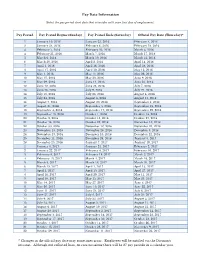
Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

2021 Park Hours
Holiday World & Splashin' Safari 2021 Operating Calendar May, 2021 Holiday World Hours Splashin' Safari Hours Special Events Saturday, May 1 Holiday World & Splashin' Safari are closed Sunday, May 2 Holiday World & Splashin' Safari are closed Monday, May 3 Holiday World & Splashin' Safari are closed Tuesday, May 4 Holiday World & Splashin' Safari are closed Wednesday, May 5 Holiday World & Splashin' Safari are closed Thursday, May 6 Holiday World & Splashin' Safari are closed Friday, May 7 Holiday World & Splashin' Safari are closed Saturday, May 8 Platinum Season Pass Preview Day: Holiday World is open from 10 AM until 6 PM Splashin' Safari is Closed Platinum Season Passholder Party Sunday, May 9 Passholder Preview Day: Holiday World is open from 10 AM until 6 PM Splashin' Safari is Closed Monday, May 10 Holiday World & Splashin' Safari are closed Tuesday, May 11 Holiday World & Splashin' Safari are closed Wednesday, May 12 Holiday World & Splashin' Safari are closed Thursday, May 13 Holiday World & Splashin' Safari are closed Friday, May 14 Holiday World & Splashin' Safari are closed for a private outing Saturday, May 15 Holiday World is open from 10 AM until 4 PM Splashin' Safari is Closed Sunday, May 16 Holiday World is open from 10 AM until 4 PM Splashin' Safari is Closed Monday, May 17 Holiday World & Splashin' Safari are closed Tuesday, May 18 Holiday World & Splashin' Safari are closed Wednesday, May 19 Holiday World & Splashin' Safari are closed Thursday, May 20 Holiday World & Splashin' Safari are closed Friday, May 21 Holiday -

Weekly Report for Deaths Attributed to Covid-19 Issued August 13, 2021
BUREAU OF HEALTH STATISTICS AND REGISTRIES WEEKLY REPORT FOR DEATHS ATTRIBUTED TO COVID-19 ISSUED AUGUST 13, 2021 DEATHS REGISTERED: 27,957 This report is based on COVID-19 deaths that occurred in Pennsylvania. This information has been extracted from death records registered with the Department’s Vital Records Program as of 11:59 pm on November 26, 2020. Please see State Registrar Notice (SRN) 2020-13 for detailed information regarding the reporting of these deaths. These deaths meet the CDC’s National Center for Health Statistics guidance.1 Death records included in this report include laboratory confirmed COVID-19 deaths and clinically confirmed COVID-19 deaths.2 This death data information is considered provisional information. Due to the time associated with reporting deaths, recent death data is incomplete and only reflected in the figures below if the death record is complete. Death data will continue to be updated as additional COVID-19 deaths are registered and amended with the Vital Records Program. GENDER Gender # of Deaths Female 13,714 Male 14,243 1 https://www.cdc.gov/nchs/nvss/vsrr/covid19/tech_notes.htm Coronavirus disease deaths are identified when coronavirus disease 2019 or COVID-19 are reported as a cause that contributed to death on the death record. These can include laboratory confirmed cases, as well as cases without laboratory confirmation. 2 This report only includes deaths among individuals who may have tested positive for COVID-19 if the death record meets the criteria listed in footnote 1 above. RACE Race # of Deaths Asian 461 African American 3,548 White 22,973 Multiple/Other 975 ETHNICITY Ethnicity # of Deaths Hispanic 1,168 Not Hispanic 26,764 Unknown 25 Pennsylvania’s Weekly Report of Deaths Attributed to COVID-19 Page 2 August 13, 2021 AGE Age # of Deaths <0-4 * 5-9 * 10-14 * 15-19 15 20-24 18 25-29 37 30-34 64 35-39 91 40-44 171 45-49 298 50-54 557 55-59 940 60-64 1,655 65-69 2,368 70-74 3,109 75-79 3,527 80-84 4,050 85-89 4,554 90-94 4,223 95-99 1,899 100-104 351 *Counts less than 5 are suppressed. -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3, -

Academic Calendar 2021-2022 Fall 2021 Semester Classes Begin
Academic Calendar 2021-2022 Fall 2021 Semester Classes Begin (Main and 7.5wk1 Session) Mon, August 23 Convocation Thu, August 26 Labor Day Holiday Break (no classes) Mon, September 6 Mercy Day Mass Thu, September 23 Fall Break (no classes) Mon-Tue, October 11-12 7.5wk1 Session Ends Mon, October 18 7.5wk2 Session Begins Tue, October 19 Thanksgiving Holiday Break (no classes) Wed-Sun, November 24-Nov 28 Study Day (no classes) Tue, December 7 Final Assessments (Main and 7.5wk2 Session) Wed-Tue, December 8-14 (Snow Date for Final Assessments) Wed, December 15 For Saturday MBA: 7.5WK1: 8/28, 9/11, 9/18, 9/25, 10/2, 10/9, 10/16 7.5WK2: 10/23, 10/30, 11/6, 11/13, 11/20, 12/4, 12/11 Winter 2022 Session Classes Meet: Wed Dec 15, Thu Dec 16, Fri Dec 17, Mon Dec 20, Tue Dec 21, Mon Jan 3-Fri Jan 7, Mon Jan 10-Fri Jan 14. Final Assessments Fri Jan 14. Spring 2022 Semester Classes Begin (Main and 7.5wk1 Session) Tue, January 18 Mass of the Holy Spirit Tue, February 8 Spring Break (no classes) Sun-Sat, Feb 27 – March 5 7.5wk1 Session Ends Mon, March 14 7.5wk2 Session Begins Tue, March 15 Critical Concerns Week Mon-Thu, March 21-24 Easter Break (no classes) Thurs-Mon, April 14 – April 18 Follow Monday Class Schedule Wed, April 20 Faculty Development Day / Study Day (no classes) Tue, May 3 Classes Resume Wed-Fri, May 4–6 Final Assessments (Main and 7.5wk2 Session) Sat-Fri, May 7-13 Baccalaureate Mass, Commencement Ceremony, Reception Wed, May 18 For Saturday MBA: 7.5WK1: 1/22, 1/29, 2/5, 2/12, 2/19, 2/26, 3/12 7.5WK2: 3/19, 3/26, 4/2, 4/9, 4/23, 4/30,