Appendix B. Dose Calculations 45
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pelagic Phytoplankton Community Change‐Points Across
Freshwater Biology (2017) 62, 366–381 doi:10.1111/fwb.12873 Pelagic phytoplankton community change-points across nutrient gradients and in response to invasive mussels † † , ‡ KATYA E. KOVALENKO*, EUAN D. REAVIE*, J. DAVID ALLAN , MEIJUN CAI*, SIGRID D. P. SMITH AND LUCINDA B. JOHNSON* *Natural Resources Research Institute, University of Minnesota Duluth, Duluth, MN, U.S.A. † School of Natural Resources and Environment, University of Michigan, Ann Arbor, MI, U.S.A. SUMMARY 1. Phytoplankton communities can experience nonlinear responses to changing nutrient concentrations, but the nature of species shifts within phytoplankton is not well understood and few studies have explored responses of pelagic assemblages in large lakes. 2. Using pelagic phytoplankton data from the Great Lakes, we assessed phytoplankton assemblage change-point responses to nutrients and invasive Dreissena, characterising community responses in a multi-stressor environment and determine whether species responses to in situ nutrients can be approximated from nutrient loading. 3. We demonstrate assemblage shifts in phytoplankton communities along major stressor gradients, particularly prominent in spring assemblages, providing insight into community thresholds at the lower end of the phosphorus gradient and species-stressor responses in a multi-stressor environment. We show that responses to water nutrient concentrations could not be estimated from large-scale nutrient loading data likely due to lake-specific retention time and long-term accumulation of nutrients. 4. These findings highlight the potential for significant accumulation of nitrates in ultra-oligotrophic systems, nonlinear responses of phytoplankton at nutrient concentrations relevant to current water quality standards and system-specific (e.g. lake or ecozone) differences in phytoplankton responses likely due to differences in nutrient co-limitation and effects of dreissenids. -

Goose Lake Nutrient Study (Marquette County, Michigan)
MI/DEQ/WD-04/013 Goose Lake Nutrient Study (Marquette County, Michigan) Prepared by: White Water Associates, Inc. 429 River Lane Amasa, Ml 49903 (SUBCONTRACTOR) Great Lakes Environmental Center 739 Hastings Street Traverse City, Ml 49686 (PRIME CONTRACTOR) Prepared for: Michigan Department of Environmental Quality Water Division Lansing, Michigan 48933-7773 Lead Staff Person: Sarah Walsh View of Goose Lake looking northwest from the Goose Lake Outlet (Marquette County). Photo by D Premo Contract Number: 071B1001643, Project Number: 03-02 Date: December 31, 2003 Goose Lake Nutrient Study (Marquette County, Michigan) Prepared by: White 'Nater Associates, Inc. 429 River Lane Amasa, rv11 49903 (SUBCONTRACTOR) Great Lakes Environmental Center 739 Hastings Street Traverse City, Ml 49686 (PRIME CONTRACTOR) Contacts: Dean B. Premo, Ph.D., White Water Associates Phone: (906) 822-7889; Fax: (906) 822-7977 E-mail: [email protected] Dennis McCauley, Great Lakes Environmental Center Phone. (231) 941-2230; Fax: (231) 941-2240 E-mail: [email protected] Prepared for: Michigan Department of Environmental Quality Water Division Lansing, Michigan 48933-7773 Lead Staff Person: Sarah Walsh Contract Number: 07181001643 Project Number: 03-02 Date: December 31, 2003 Goose Lake Nutrient Study (Marquette County, Michigan) Fieldwork: Dean Premo, Senior Ecologist David Tiller, Field Biologist Report: Dean Premo, Ph.D., Senior Ecologist Kent Premo, M.S., Technical Support Scientist Bette Premo, Ph.D., Limnologist Cite as: Premo, Dean, Kent Premo, and Bette Premo. 2003. Goose Lake Nutrient Study (Marquette County, Michigan). White Water Associates, Inc. Contents of Appendix A - Exhibits Exhibit 1. Map of the Goose Lake Study Landscape and Four Sampling Stat(ons. -

Program and Abstracts.Docx
The 2020 Atlantic Salmon Ecosystems Forum Time flies – Atlantic salmon as an endangered species twenty years later… January 14-15, 2020 Orono, Maine USA University of Maine, Wells Conference Center Recognizing the International Year of the Salmon (focus) in 2019, watch for activities extending into 2020 2020 Atlantic Salmon Ecosystems Forum Schedule At A Glance Begin End January 14, 2020 7:00 8:00 REGISTRATION - Refreshments provided 8:00 8:05 Housekeeping Rory Saunders, NOAA Fisheries 8:05 8:25 Welcome to the 2020 ASEF Sam Rauch, Deputy Assistant Administrator for Regulatory Programs, NOAA Fisheries 8:25 9:00 Sustainability as a framework for rethinking approaches to salmon, society and solutions. David Hart, Director, Senator George J. Mitchell Center for Sustainability Solutions 9:00 9:05 Session I: 20 Years of Experience Guiding our Future (Part 1 of 2) Joshua Royte, The Nature Conservancy, Moderator 9:05 9:25 Reflections on Penobscot River Atlantic Salmon: Before and After Listing as an Endangered Species Edward T Baum, Maine Atlantic Sea-Run Salmon Commission (Retired) 9:25 9:45 From North America to West Greenland and Beyond: management of Atlantic salmon in the North Atlantic Martha Jean Robertson, Fisheries and Oceans Canada, Newfoundland and Labrador, Canada 9:45 10:15 BREAK - refreshments provided 10:15 10:25 Science for comfort or conservation- how do we inform and avoid action on fish passage? Joseph D Zydlewski, U.S. Geological Survey, Maine Cooperative Fish and Wildlife Research 10:25 10:35 Using decision support tools to plan for salmon restoration Erik H Martin, The Nature Conservancy 10:35 10:45 6 ½ & 19 years Maintaining and Perfecting SHARE’s Mission Focus Steven D. -

American Eel Anguilla Rostrata
COSEWIC Assessment and Status Report on the American Eel Anguilla rostrata in Canada SPECIAL CONCERN 2006 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2006. COSEWIC assessment and status report on the American eel Anguilla rostrata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 71 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge V. Tremblay, D.K. Cairns, F. Caron, J.M. Casselman, and N.E. Mandrak for writing the status report on the American eel Anguilla rostrata in Canada, overseen and edited by Robert Campbell, Co-chair (Freshwater Fishes) COSEWIC Freshwater Fishes Species Specialist Subcommittee. Funding for this report was provided by Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur l’anguille d'Amérique (Anguilla rostrata) au Canada. Cover illustration: American eel — (Lesueur 1817). From Scott and Crossman (1973) by permission. ©Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/458-2006E-PDF ISBN 0-662-43225-8 Recycled paper COSEWIC Assessment Summary Assessment Summary – April 2006 Common name American eel Scientific name Anguilla rostrata Status Special Concern Reason for designation Indicators of the status of the total Canadian component of this species are not available. -

Change of Phytoplankton Composition and Biodiversity in Lake Sempach Before and During Restoration
Hydrobiologia 469: 33–48, 2002. S.A. Ostroumov, S.C. McCutcheon & C.E.W. Steinberg (eds), Ecological Processes and Ecosystems. 33 © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Change of phytoplankton composition and biodiversity in Lake Sempach before and during restoration Hansrudolf Bürgi1 & Pius Stadelmann2 1Department of Limnology, ETH/EAWAG, CH-8600 Dübendorf, Switzerland E-mail: [email protected] 2Agency of Environment Protection of Canton Lucerne, CH-6002 Lucerne, Switzerland E-mail: [email protected] Key words: lake restoration, biodiversity, evenness, phytoplankton, long-term development Abstract Lake Sempach, located in the central part of Switzerland, has a surface area of 14 km2, a maximum depth of 87 m and a water residence time of 15 years. Restoration measures to correct historic eutrophication, including artificial mixing and oxygenation of the hypolimnion, were implemented in 1984. By means of the combination of external and internal load reductions, total phosphorus concentrations decreased in the period 1984–2000 from 160 to 42 mg P m−3. Starting from 1997, hypolimnion oxygenation with pure oxygen was replaced by aeration with fine air bubbles. The reaction of the plankton has been investigated as part of a long-term monitoring program. Taxa numbers, evenness and biodiversity of phytoplankton increased significantly during the last 15 years, concomitant with a marked decline of phosphorus concentration in the lake. Seasonal development of phytoplankton seems to be strongly influenced by the artificial mixing during winter and spring and by changes of the trophic state. Dominance of nitrogen fixing cyanobacteria (Aphanizomenon sp.), causing a severe fish kill in 1984, has been correlated with lower N/P-ratio in the epilimnion. -
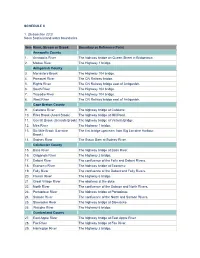
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

Equilibrium Analyses of the Recovery Feasibility of Four Atlantic Salmon (Salmo Salar) Populations in Nova Scotia and Southwest New Brunswick
ICES CM 2008/N:11 Equilibrium analyses of the recovery feasibility of four Atlantic salmon (Salmo salar) populations in Nova Scotia and Southwest New Brunswick Authors: A. Jamie F. Gibson1, Ross A. Jones2, Peter G. Amiro3 and Heather D. Bowlby4 1Department of Fisheries and Oceans, P.O. Box 1006, Dartmouth, N.S., Canada, B2Y 4A2; Tel: 902-426-3136; email: [email protected] 2Department of Fisheries and Oceans, 343 Université Ave, Moncton, N.B., Canada, E1C 9B6; Tel: 506-851-6441; email: [email protected] 3Department of Fisheries and Oceans, P.O. Box 1006, Dartmouth, N.S., Canada, B2Y 4A2; Tel: 902-426-8104; email: [email protected] 4Department of Fisheries and Oceans, P.O. Box 1006, Dartmouth, N.S., Canada, B2Y 4A2; Tel: 902-426-5836; email: [email protected] Abstract: Abundances of Atlantic salmon in rivers along the Atlantic Coast of Nova Scotia and around the Bay of Fundy, Canada, have declined precipitously during the last two decades. Equilibrium analyses were carried out on four populations in this region in order to evaluate the relationship between threats to these populations, their recovery potential, and the expected population response to recovery actions. Equilibrium models split the life cycle of a species into two or more parts and determine the population size at which the rates in each part of the life cycle are balanced such that the population does not increase or decrease in size. By varying the life history parameters in a way that represents the expected response to a human activity and examining the resulting change in equilibrium population size, the effects of the activity on the population can be evaluated. -

Ices Wgnas 2012 Addendum
ICES WGNAS 2012 ADDENDUM ICES Advisory Committee ICES CM 2012/ACOM:09 ICES COMPILATION OF MICROTAGS, FINCLIP AND EXTERNAL TAG RELEASES 2011 BY THE WORKING GROUP ON NORTH ATLANTIC SALMON 26 MARCH–4 APRIL 2012 COPENHAGEN, DENMARK International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2012. ICES Compilation of Microtags, Finclip and External Tag Releases 2011 by the Working Group on North Atlantic Salmon. ICES WGNAS 2012 ADDENDUM 26 March–4 April 2012. 31 pp. For permission to reproduce material from this publication, please apply to the General Secretary. This document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the view of the Council. © 2012 International Council for the Exploration of the Sea ICES WGNAS 2012 ADDENDUM | i Contents 1 Terms of Reference ........................................................................................................ 2 2 Summary table ............................................................................................................... 3 3 Number of tags and marks applied to Atlantic salmon by country for 2011 ................................................................................................................................... 5 3.1 Canada .................................................................................................................. -

South Western Nova Scotia
Netukulimk of Aquatic Natural Life “The N.C.N.S. Netukulimkewe’l Commission is the Natural Life Management Authority for the Large Community of Mi’kmaq /Aboriginal Peoples who continue to reside on Traditional Mi’Kmaq Territory in Nova Scotia undisplaced to Indian Act Reserves” P.O. Box 1320, Truro, N.S., B2N 5N2 Tel: 902-895-7050 Toll Free: 1-877-565-1752 2 Netukulimk of Aquatic Natural Life N.C.N.S. Netukulimkewe’l Commission Table of Contents: Page(s) The 1986 Proclamation by our late Mi’kmaq Grand Chief 4 The 1994 Commendation to all A.T.R.A. Netukli’tite’wk (Harvesters) 5 A Message From the N.C.N.S. Netukulimkewe’l Commission 6 Our Collective Rights Proclamation 7 A.T.R.A. Netukli’tite’wk (Harvester) Duties and Responsibilities 8-12 SCHEDULE I Responsible Netukulimkewe’l (Harvesting) Methods and Equipment 16 Dangers of Illegal Harvesting- Enjoy Safe Shellfish 17-19 Anglers Guide to Fishes Of Nova Scotia 20-21 SCHEDULE II Specific Species Exceptions 22 Mntmu’k, Saqskale’s, E’s and Nkata’laq (Oysters, Scallops, Clams and Mussels) 22 Maqtewe’kji’ka’w (Small Mouth Black Bass) 23 Elapaqnte’mat Ji’ka’w (Striped Bass) 24 Atoqwa’su (Trout), all types 25 Landlocked Plamu (Landlocked Salmon) 26 WenjiWape’k Mime’j (Atlantic Whitefish) 26 Lake Whitefish 26 Jakej (Lobster) 27 Other Species 33 Atlantic Plamu (Salmon) 34 Atlantic Plamu (Salmon) Netukulimk (Harvest) Zones, Seasons and Recommended Netukulimk (Harvest) Amounts: 55 SCHEDULE III Winter Lake Netukulimkewe’l (Harvesting) 56-62 Fishing and Water Safety 63 Protecting Our Community’s Aboriginal and Treaty Rights-Community 66-70 Dispositions and Appeals Regional Netukulimkewe’l Advisory Councils (R.N.A.C.’s) 74-75 Description of the 2018 N.C.N.S. -

The Geographic Distribution and Aspects of the Parasite/Host
Geographic distribution and aspects of the parasite/host relationships of the invasive swim bladder parasite Anguillicoloides crassus infecting American eel (Anguilla rostrata) in mainland Nova Scotia and New Brunswick By Dollie M. Campbell A Thesis submitted to Saint Mary’s University, Halifax, Nova Scotia in Partial Fulfillment of the Requirements for the Degree of Master of Science in Applied Science April 2014, Halifax, Nova Scotia © Dollie M. Campbell 2014 Approved: Dr. David Cone Supervisor Approved: Dr. David Cairns External examiner Approved: Dr. Katherine Jones Committee member Approved: Dr. Ron Russell Committee member Approved: Dr. Susan Bjornson Graduate Studies Rep. Date: April 15, 2014 Table of Contents ABSTRACT iii ACKNOWLEDGEMENTS iv LIST OF TABLES v LIST OF FIGURES vi - ix INTRODUCTION 1 - 7 MATERIALS AND METHODS Eel collection 7 - 9 Eel necropsies 9 - 10 Data analysis 10 - 11 RESULTS 11 - 13 Parasite host relationship and diet 12 - 13 DISCUSSION 14 - 19 REFERANCES 20 - 26 APPENDIX 1. 0 : American eel otolith preparation and ageing 62 - 66 ii Abstract Geographic distribution and aspects of the parasite/host relationships of the invasive swim bladder parasite Anguillicoloides crassus infecting American eel (Anguilla rostrata) in mainland Nova Scotia and New Brunswick Dollie M. Campbell Between 2008-2013, 1,981 eels were collected from 174 localities throughout mainland Nova Scotia and New Brunswick and necropsied for the swim bladder nematode Anguillicoloides crassus. Overall prevalence of A. crassus was 4 % with a mean intensity of 3.8 ± 8 SD (1-63 parasites). The Southern Uplands, Gulf of St. Lawrence and the Bay of Fundy regions were all identified as having rivers with eels infected with the nematode. -

Discussion Paper on Brackish Urban Lake Water Quality in South East Queensland Catalano, C.L
Discussion Paper on Brackish Urban Lake Water Quality in South East Queensland Catalano, C.L. 1, Dennis, R.B. 2, Howard, A.F.3 Cardno Lawson Treloar12, Cardno3 Abstract Cardno has been involved in the design and monitoring of a number of urban lakes and canal systems within south east Queensland for over 30 years. There are now many urban lakes in South East Queensland and the majority have been designed on the turnover or lake flushing concept, whereby it is considered that, if the lake is flushed within a nominal timeframe, then there is a reasonable expectation that the lake will be of good health. The designs have predominately been based on a turnover or residence time of around 20-30 days and some of the lakes reviewed are now almost 30 years old. This paper reviews this methodology against collected water quality data to provide comment on the effectiveness of this method of design for brackish urban lakes in South East Queensland and also to indicate where computational modelling should be used instead of, or to assist with, this methodology. 1. Introduction Lakeside developments are very popular in South-East Queensland. The lake is generally artificial, created out of a modification of an existing watercourse or lowland area for a source of fill for the surrounding residential construction. They are used to provide visual and recreational amenity, sometimes including boat navigation and mooring areas, and can also serve as detention basins and water quality polishing devices. As with anypermanent water feature, they inevitability also become an aquatic habitat. -

Newman Lake Total Phosphorus TMDL
Newman Lake Total Phosphorus Total Maximum Daily Load Water Quality Improvement Report November 2007 Publication Number 06-10-045 Newman Lake Total Phosphorus Total Maximum Daily Load Water Quality Improvement Report Prepared by: Anthony J. Whiley and Ken Merrill Washington State Department of Ecology Water Quality Program November 2007 Publication Number 06-10-045 You can print or download this document from our Web site at http://www.ecy.wa.gov/biblio/0610045.html For more information contact: Department of Ecology Water Quality Program Watershed Management Section P.O. Box 47600 Olympia, WA 98504-7600 Telephone: 360-407-6404 Headquarters (Lacey) 360-407-6000 Regional Whatcom Pend San Juan Office Oreille location Skagit Okanogan Stevens Island Northwest Central Ferry 425-649-7000 Clallam Snohomish 509-575-2490 Chelan Jefferson Spokane K Douglas i Bellevue Lincoln ts Spokane a Grays p King Eastern Harbor Mason Kittitas Grant 509-329-3400 Pierce Adams Lacey Whitman Thurston Southwest Pacific Lewis 360-407-6300 Yakima Franklin Garfield Wahkiakum Yakima Columbia Walla Cowlitz Benton Asotin Skamania Walla Klickitat Clark Persons with a hearing loss can call 711 for Washington Relay Service. Persons with a speech disability can call 877-833-6341. If you need this publication in an alternate format, please call the Water Quality Program at 360-407-6404. Persons with hearing loss can call 711 for Washington Relay Service. Persons with a speech disability can call 877-833-6341 Table of Contents List of Figures and Tables..............................................................................................................iii