Eriophyllum Confertiflorum (DC.) A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Likely to Have Habitat Within Iras That ALLOW Road
Item 3a - Sensitive Species National Master List By Region and Species Group Not likely to have habitat within IRAs Not likely to have Federal Likely to have habitat that DO NOT ALLOW habitat within IRAs Candidate within IRAs that DO Likely to have habitat road (re)construction that ALLOW road Forest Service Species Under NOT ALLOW road within IRAs that ALLOW but could be (re)construction but Species Scientific Name Common Name Species Group Region ESA (re)construction? road (re)construction? affected? could be affected? Bufo boreas boreas Boreal Western Toad Amphibian 1 No Yes Yes No No Plethodon vandykei idahoensis Coeur D'Alene Salamander Amphibian 1 No Yes Yes No No Rana pipiens Northern Leopard Frog Amphibian 1 No Yes Yes No No Accipiter gentilis Northern Goshawk Bird 1 No Yes Yes No No Ammodramus bairdii Baird's Sparrow Bird 1 No No Yes No No Anthus spragueii Sprague's Pipit Bird 1 No No Yes No No Centrocercus urophasianus Sage Grouse Bird 1 No Yes Yes No No Cygnus buccinator Trumpeter Swan Bird 1 No Yes Yes No No Falco peregrinus anatum American Peregrine Falcon Bird 1 No Yes Yes No No Gavia immer Common Loon Bird 1 No Yes Yes No No Histrionicus histrionicus Harlequin Duck Bird 1 No Yes Yes No No Lanius ludovicianus Loggerhead Shrike Bird 1 No Yes Yes No No Oreortyx pictus Mountain Quail Bird 1 No Yes Yes No No Otus flammeolus Flammulated Owl Bird 1 No Yes Yes No No Picoides albolarvatus White-Headed Woodpecker Bird 1 No Yes Yes No No Picoides arcticus Black-Backed Woodpecker Bird 1 No Yes Yes No No Speotyto cunicularia Burrowing -

© 2020 Theodore Payne Foundation for Wild Flowers & Native Plants. No
May 8, 2020 Theodore Payne Foundation’s Wild Flower Hotline is made possible by donations, memberships and sponsors. You can support TPF by shopping the online gift store as well. A new, pay by phone, contactless plant pickup system is now available. Details here. Widespread closures remain in place. If you find an accessible trail, please practice social distancing. The purpose for the Wild Flower Hotline now is NOT to send you to localities for wild flower viewing, but to post photos that assure you—virtually—that California’s wild spaces are still open for business for flowers and their pollinators. LA County’s Wildlife Sanctuaries are starting to dry up from the heat. This may be the last week to see flowers at Jackrabbit Flats and Theodore Payne Wildlife Sanctuaries near Littlerock in the high desert. Yellow is the dominant color with some pink and white scattered about. Parry’s linanthus (Linanthus parryae) and Bigelow’s coreopsis (Leptosyne bigelovii), are widespread. Small patches of goldfields (Lasthenia californica), and Mojave sun cups (Camissonia campestris) are still around. If you are visiting around dusk, the evening snow (Linanthus dichotomus) open up and put on a display that lives up to its name. Strewn around are Pringle’s woolly sunflower (Eriophyllum pringlei), white tidy tips (Layia glandulosa), owl’s clover (Castilleja sp.) and desert dandelion (Malacothrix glabrata). Underneath the creosote bushes, lacy phacelia (Phacelia tanacetifolia) is seeking out some shade. Theodore Payne Sanctuary has all these flowers, and because it has more patches of sandy alluvial soils, has some cute little belly flowers like Wallace’s wooly daisy (Eriophyllum wallacei) and purple mat (Nama demissa) too. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Idell Weydemeyer's Native Plants TREES SHRUBS & SUBSHRUBS
Idell Weydemeyer’s Native Plants 11-04 Note: • All plants on here are drought resistant except those originating in moist areas. Some will die if given summer water. Sun required unless shade is mentioned. • “LOCAL” means found growing in Idell’s garden or within 100 yards; “Local” means growing within ten miles from the garden. • Thr & Endgr refers to plant posting on Threatened or Endangered List. • There is disagreement among authors as to the range or locations for various plants. TREES Native Plant Common Name Location Aesculus californica California Buckeye LOCAL; Central Coast Ranges to Sierras & Tehachapis; in woodlands, forests & chaparral; on dry slopes & canyons near water; takes clay; deciduous by July or August Arbutus menziesii Madrone Coast Ranges from Baja to British Columbia & N. Sierras; wooded slopes & canyons; full sun to high afternoon shade, well drained acidic soil Calocedrus decurrens Incense Cedar Oregon to Baja, Nevada & Utah; sandy to clay soil Cercidium floridum Palo Verde California, Arizona, Mexico & Central America; Southern California desert in creosote bush Blue Palo Verde scrub & Colorado Desert (in CA) below 3,000 feet; by dry creeks with water in summer & winter, perfect drainage, no summer water; deciduous part of year Pinus (possibly jeffreyi) Jeffrey Pine Platanus racemosa California Sycamore Coast Ranges & foothills in warmer parts of CA; along creeks; drought tolerant only with high Western Sycamore water table or along coast, tolerates full sun, part shade, seasonal flooding, sand & clay soil; deciduous in fall & winter Populus Cottonwood Regular water; deciduous in winter Prunus ilicifolia Holly-leaved Cherry Coast Ranges from Napa southward into Mexico & to Santa Catalina & San Clement Islands; on dry slopes & flats of foothills Prunus subcordata Klamath Plum Southern California Sierras, Northern California into Oregon; some moisture; deciduous in Sierra Plum winter Prunus virginiana (probably demissa) Chokecherry Most of the West into S. -

Idaho PM Technical Note 2B (Revise): Plants for Pollinators in the Inland Northwest
TECHNICAL NOTE USDA – Natural Resources Conservation Service Boise, Idaho - Spokane, Washington ______________________________________________________________________________ TN PLANT MATERIALS NO. 2B OCTOBER 2011 REVISION Plants for Pollinators in the Inland Northwest Dan Ogle, Plant Materials Specialist, NRCS, Boise, Idaho Pamela Pavek, Agronomist, NRCS Plant Materials Center, Pullman, Washington Richard Fleenor, Plant Materials Specialist, NRCS, Spokane, Washington Mark Stannard, Manager, NRCS Plant Materials Center, Pullman, Washington Tim Dring, State Biologist, NRCS, Spokane, Washington Jim Cane, Bee Biology and Systematics Lab, ARS, Logan, Utah Karen Fullen, State Biologist, NRCS, Boise, Idaho Loren St. John, Manager, NRCS Plant Materials Center, Aberdeen, Idaho Derek Tilley, Agronomist, NRCS Plant Materials Center, Aberdeen, Idaho Brownbelted bumble bee (Bombus griseocollis) visiting a blanketflower (Gaillardia aristata). Pamela Pavek The purpose of this Technical Note is to provide guidance for the design and implementation of conservation plantings to enhance habitat for pollinators including: bees, wasps, butterflies, moths and hummingbirds. Plant species included in this document are adapted to the Inland Northwest, which encompasses northern Idaho, northeastern Oregon and eastern Washington. For species adapted to southern Idaho, southeastern Oregon, northern Nevada and northern Utah, refer to Idaho Plant Materials Technical Note 2A. For lists of species adapted to western Washington and western Oregon, refer to the Oregon -

Penstemon Palmeri Family: Scrophulariaceae Notes: Palmer’S Penstemon Is a Perennial Herb to a Slightly Woody Subshrub 5 to 14 Dm Tall with a Thick Crown
Common name: Palmer’s penstemon Scientific name: Penstemon palmeri Family: Scrophulariaceae Notes: Palmer’s penstemon is a perennial herb to a slightly woody subshrub 5 to 14 dm tall with a thick crown. The plant is glabrous and glaucous with fleshy leaves. The leaves are dentate with the upper ones sometimes triangular. The flowers range from white to lavender-pink. They have several stalked flowers or flower clusters that are borne in the axils of the upper leaves or leaf-like bracts. The tubular corolla is strongly to distinctly two-lipped at the mouth with a two-lobed upper lip and a three-lobed lower lip. There are 4 anther-bearing (fertile) stamens and a single sterile stamen or staminodia that is often hairy at the tip. The fruit is a many- seeded capsule. Common name: Lewis flax Scientific name: Linum lewisii Family: Linaceae Notes: Flax plants have many narrow, small, alternate (rarely opposite), simple and entire leaves that are sessile (lacking stalks) on the stems. The perfect and regular, generally showy flowers are borne in racemes or cymes. The sepals, petals, and stamens are five, the fruit a capsule, and the seeds in most species are mucilaginous when wet. In general, flax is an annual or short-lived, semi-evergreen perennial forb, sometimes semi-woody at base with attractive flowers ranging from white to blue to yellow to red in color. Common name: Rocky Mountain penstemon Scientific name: Penstemon strictus Family: Scrophulariaceae Notes: Penstemon strictus is a perennial herb growing 12 to 36 in tall. It has one to few stems arising from a thick crown. -

Sierra Azul Wildflower Guide
WILDFLOWER SURVEY 100 most common species 1 2/25/2020 COMMON WILDFLOWER GUIDE 2019 This common wildflower guide is for use during the annual wildflower survey at Sierra Azul Preserve. Featured are the 100 most common species seen during the wildflower surveys and only includes flowering species. Commonness is based on previous surveys during April for species seen every year and at most areas around Sierra Azul OSP. The guide is a simple color photograph guide with two selected features showcasing the species—usually flower and whole plant or leaf. The plants in this guide are listed by Color. Information provided includes the Latin name, common name, family, and Habit, CNPS Inventory of Rare and Endangered Plants rank or CAL-IPC invasive species rating. Latin names are current with the Jepson Manual: Vascular Plants of California, 2012. This guide was compiled by Cleopatra Tuday for Midpen. Images are used under creative commons licenses or used with permission from the photographer. All image rights belong to respective owners. Taking Good Photos for ID: How to use this guide: Take pictures of: Flower top and side; Leaves top and bottom; Stem or branches; Whole plant. llama squash Cucurbitus llamadensis LLAMADACEAE Latin name 4.2 Shrub Common name CNPS rare plant rank or native status Family name Typical bisexual flower stigma pistil style stamen anther Leaf placement filament petal (corolla) sepal (calyx) alternate opposite whorled pedicel receptacle Monocots radial symmetry Parts in 3’s, parallel veins Typical composite flower of the Liliy, orchid, iris, grass Asteraceae (sunflower) family 3 ray flowers disk flowers Dicots Parts in 4’s or 5’s, lattice veins 4 Sunflowers, primrose, pea, mustard, mint, violets phyllaries bilateral symmetry peduncle © 2017 Cleopatra Tuday 2 2/25/2020 BLUE/PURPLE ©2013 Jeb Bjerke ©2013 Keir Morse ©2014 Philip Bouchard ©2010 Scott Loarie Jim brush Ceanothus oliganthus Blue blossom Ceanothus thyrsiflorus RHAMNACEAE Shrub RHAMNACEAE Shrub ©2003 Barry Breckling © 2009 Keir Morse Many-stemmed gilia Gilia achilleifolia ssp. -

Kern County, California
5564 Federal Register / Vol. 77, No. 23 / Friday, February 3, 2012 / Notices DEPARTMENT OF THE INTERIOR Tehachapi Upland Draft MSHCP/SEIS incidental to, and not the purpose of, Comments. carrying out an otherwise lawful Fish and Wildlife Service Hard bound copies of the SDEIS, TU activity. Regulations governing permits MSHCP, and IA are available for for threatened species and endangered [FWS–R8–2011–N270; FF08E00000– viewing at the following locations: species, respectively, are at 50 CFR FXES11120800000F2–112] 1. U.S. Fish and Wildlife Service, 17.32 and 50 CFR 17.22. Tehachapi Uplands Multiple Species 2493 Portola Road, Suite B, Ventura, CA Although take of listed plant species Habitat Conservation Plan; Kern 93003. is not prohibited under the Act, and County, CA 2. Kern County Library, Frazier Park therefore cannot be authorized by an Branch, 3732 Park Drive, Frazier Park, incidental take permit, plant species AGENCY: Fish and Wildlife Service, CA 93225. may be included on a permit in Interior. FOR FURTHER INFORMATION CONTACT: recognition of the conservation benefits ACTION: Notice of availability of Steve Kirkland, Fish and Wildlife provided to them by a habitat Supplemental Draft Environmental Biologist, U.S. Fish and Wildlife conservation plan. All species included Impact Statement. Service, at 805–644–1766. on an incidental take permit would receive assurances under the Service’s SUPPLEMENTARY INFORMATION: SUMMARY: The Fish and Wildlife Service ‘‘No Surprises’’ regulation [50 CFR (Service) announces the availability of a Introduction 17.22(b)(5) and 17.32(b)(5)]. Supplemental Draft Environmental The Applicant seeks a 50-year We have received an application for Impact Statement (SDEIS) for the incidental take permit for covered an incidental take permit covering 27 Tehachapi Uplands Multiple Species activities within 141,886 acres of listed and unlisted species that may be Habitat Conservation Plan (TU MSHCP) covered lands on Tejon Ranch lands in taken or otherwise affected by on-going and the draft TU MSHCP and Kern County, California. -

Protocol Information
Protocol Information Dave Skinner PMC Farm Manager Pullman Plant Materials Center Room 211A Hulbert Hall WSU Pullman, Washington 99163-6211 509-335-9689 509-335-2940 Fax [email protected] Family Scientific Name: Asteraceae Family Common Name: Sunflower Scientific Name: Eriophyllum lanatum (Pursh) Forbes Common Name: Oregon sunshine Species Code: ERLA6 Ecotype: south of Moscow, ID General Distribution: Dry, open, often rocky places in grasslands and dry forest from southern British Columbia to California and east to Montana, Wyoming, and Utah. Known Invasiveness: not invasive Propagation Goal: Plants Propagation Method: Seed Product Type: Container (plug) Stock Type: 10 cu. in. Time To Grow: 4 Months Target Specifications: Tight root plug in container. Propagule Collection: Fruit is an achene which ripens in mid to late July. Seed is dark grayish brown to nearly black in color. The pappus is reduced to short scales or is lacking entirely and the achene is not windborne. Seed will hold in the inflorescence longer than the seed of many other members of Asteraceae, but will shatter within a week or so of ripening. Small amounts are collected by hand and stored in paper bags or envelopes at room temperature until cleaned. 818,000 seeds/lb (Hassell et al 1996). Propagule Processing: Small amounts are rubbed to free the seed, then cleaned with an air column separator. Larger amounts can probably be threshed with a hammermill, then cleaned with air screen equipment. Clean seed is stored in controlled conditions at 40 degrees Fahrenheit and 40% relative humidity. Pre-Planting Treatments: Seed stored at room temperature remains viable after 8 years (Mooring 1975) but germination decreases sharply after 2 years (Mooring 2001). -
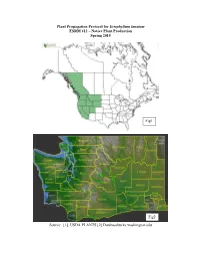
Draft Plant Propagation Protocol
Plant Propagation Protocol for Eriophyllum lanatum ESRM 412 – Native Plant Production Spring 2015 Fig1 Fig2 Source: [1]: USDA PLANTS [2]:Databaseburke.washington.edu TAXONOMY Plant Family Scientific Name Asteraceae Common Name Sunflower Species Scientific Name Scientific Name Eriophyllum lanatum (Pursh) Forbes Varieties Sub-species Eriophyllum lanatum var. achillaeoides (DC.) Jeps. Eriophyllum lanatum var. aphanactis J.T. Howell Eriophyllum lanatum var. arachnoideum (Fisch. & Avé-Lall.) Jeps. Eriophyllum lanatum var. aroceum (Greene) Jeps. Eriophyllum lanatum var. auneatum (Kellogg) Jeps. Eriophyllum lanatum var. grandiflorum (A. Gray) Jeps. Eriophyllum lanatum var. hallii Constance Eriophyllum lanatum var. integrifolium (Hook.) Eriophyllum lanatum var. lanatum (Rydb.) Jeps. Eriophyllum lanatum var. lanceolatum (Howell) Jeps. Eriophyllum lanatum var. leucophyllum (DC.) W.R. Carter Eriophyllum lanatum var. obovatum (Greene) H.M. Hall Cultivar Common Synonym(s) Common Name(s) Sunflower, Oregon sunshine, common woolly sunflower Species Code (as per USDA Plants ERLA6 database) GENERAL INFORMATION Geographical range Grasslands and dry forest from southern British Columbia to California and east to Montana, Wyoming, and Utah. Ecological distribution Dry, open, often rocky areas at low to mid-elevations Climate and elevation range Dry and sunny; Lowlands to mid elevations in the mountains. (Sierra Smith) Local habitat and abundance Selaginella wallacei, Allium acuminatum, Grindelia integrifolia, Achillea, Juncus, Bromus, Erodium, Centaurea, Sisymbrium, Agropyron, Anthriscus, Salix, Poa, Medicago, Nepeta, Chrysopsis (Sierra Smith) Plant strategy type / successional Long-lived herbaceous perennial. Rapid colonizer. stage Produces seed the first year. Plant characteristics Perennial herb 10-60 cm tall with several often lax stems from the base. Woolly, grey-green leaves usually lobed but may be entire, 1-8 cm long. -

Checklist of Vascular Plants of Lockwood Valley, Ventura County, California by David L
Checklist of Vascular Plants of Lockwood Valley, Ventura County, California By David L. Magney Botanical Name Common Name Family Achillea millefolium White Yarrow Asteraceae Achnatherum hymenoides Indian Ricegrass Poaceae Agoseris retrorsa Mountain Dandelion Asteraceae Allium sp. Onion Alliaceae Amsinckia menziesii var. intermedia Common Fiddleneck Boraginaceae Arctostaphylos parryana Parry Manzanita Ericaceae Argemone munita var. munita Prickly Poppy Papaveraceae Artemisa douglasiana Mugwort Asteraceae Artemisia tridentata var. tridentata Great Basin Sagebrush Asteraceae Artemisia tridentata var. vaseyana Mountain Great Basin Sagebrush Asteraceae Bloomeria crocea var. crocea Golden Stars Alliaceae Bromus tectorum var. tectorum Downy Brome Poaceae Calochortus clavatus var. pallidus ? Pale Yellow Mariposa Lily Liliaceae Calystegia malacophylla ssp. pedicellata Woolly Morning-glory Convolvulaceae Carex senta Rough Sedge Cyperaceae Chaenactis santelinoides Perennial Pincushion Asteraceae Chamaesyce albomarginata Rattlesnake Spurge Euphorbiaceae Chrysothamnus nauseosus ssp. consimilis Common Rubber Rabbitbrush Asteraceae Chrysothamnus nauseosus ssp. hololeucus White Rubber Rabbitbrush Asteraceae Cirsium occidentale var.? Thistle Asteraceae Collomia tinctoria Collomia Polemoniaceae Datisca glomerata Durango Root Datiscaceae Eleocharis acicularis acicularis Slender Spike-rush Cyperaceae Elymus elymoides elymoides Bottlebrush Squirreltail Poaceae Ephedra viridis Green Mormon Tea Ephedraceae Epilobium canum California Fuchsia Onagraceae Equisetum -

California Coastal Steppe Mixed Forest Redwood Forest Province
Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the California Coastal Steppe Mixed Forest Redwood Forest Province Along the Northern California Coast and NAPPC Table of CONTENTS Why Support Pollinators? 4 Getting Started 5 California Coastal Steppe 6 Meet the Pollinators 8 Plant Traits 10 Developing Plantings 12 Far ms 13 Public Lands 14 Home Landscapes 15 Bloom Periods 16 Plants That Attract Pollinators 18 Habitat Hints 20 This is one of several guides for Check list 22 different regions in the United States. We welcome your feedback to assist us in making the future Resources and Feedback 23 guides useful. Please contact us at [email protected] Cover: Northern California coastline by Marguerite Meyer 2 Selecting Plants for Pollinators Selecting Plants for Pollinators A Regional Guide for Farmers, Land Managers, and Gardeners In the Ecological Region of the California Coastal Steppe Mixed Forest Redwood Forest Province Along the Northern California Coast a nappc and Pollinator Partnership™ Publication This guide was funded by the National Fish and Wildlife Foundation, the C.S. Fund, the Plant Conservation Alliance, the U.S. Forest Service, and the Bureau of Land Management with oversight by the Pollinator Partnership™ (www.pollinator.org), in support of the North American Pollinator Protection Campaign (NAPPC–www.nappc.org). California Coastal Steppe - Mixed Forest - Redwood Forest Province 3 Why support pollinators? In theIr 1996 book, the Forgotten PollInators, Buchmann and Nabhan estimated that animal pollinators are needed for the “ Farming feeds reproduction of 90% of flowering plants and one third of human food crops.