Determination of Cannabidiol and Additional Cannabinoid Content in Hemp Tea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System
International Journal of Molecular Sciences Review Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System Shenglong Zou and Ujendra Kumar * Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC V6T 1Z4, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-604-827-3660; Fax: +1-604-822-3035 Received: 9 February 2018; Accepted: 11 March 2018; Published: 13 March 2018 Abstract: The biological effects of cannabinoids, the major constituents of the ancient medicinal plant Cannabis sativa (marijuana) are mediated by two members of the G-protein coupled receptor family, cannabinoid receptors 1 (CB1R) and 2. The CB1R is the prominent subtype in the central nervous system (CNS) and has drawn great attention as a potential therapeutic avenue in several pathological conditions, including neuropsychological disorders and neurodegenerative diseases. Furthermore, cannabinoids also modulate signal transduction pathways and exert profound effects at peripheral sites. Although cannabinoids have therapeutic potential, their psychoactive effects have largely limited their use in clinical practice. In this review, we briefly summarized our knowledge of cannabinoids and the endocannabinoid system, focusing on the CB1R and the CNS, with emphasis on recent breakthroughs in the field. We aim to define several potential roles of cannabinoid receptors in the modulation of signaling pathways and in association with several pathophysiological conditions. We believe that the therapeutic significance of cannabinoids is masked by the adverse effects and here alternative strategies are discussed to take therapeutic advantage of cannabinoids. Keywords: cannabinoid; endocannabinoid; receptor; signaling; central nervous system 1. Introduction The plant Cannabis sativa, better known as marijuana, has long been used for medical purpose throughout human history. -

N-Acyl-Dopamines: Novel Synthetic CB1 Cannabinoid-Receptor Ligands
Biochem. J. (2000) 351, 817–824 (Printed in Great Britain) 817 N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo Tiziana BISOGNO*, Dominique MELCK*, Mikhail Yu. BOBROV†, Natalia M. GRETSKAYA†, Vladimir V. BEZUGLOV†, Luciano DE PETROCELLIS‡ and Vincenzo DI MARZO*1 *Istituto per la Chimica di Molecole di Interesse Biologico, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy, †Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, R. A. S., 16/10 Miklukho-Maklaya Str., 117871 Moscow GSP7, Russia, and ‡Istituto di Cibernetica, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy We reported previously that synthetic amides of polyunsaturated selectivity for the anandamide transporter over FAAH. AA-DA fatty acids with bioactive amines can result in substances that (0.1–10 µM) did not displace D1 and D2 dopamine-receptor interact with proteins of the endogenous cannabinoid system high-affinity ligands from rat brain membranes, thus suggesting (ECS). Here we synthesized a series of N-acyl-dopamines that this compound has little affinity for these receptors. AA-DA (NADAs) and studied their effects on the anandamide membrane was more potent and efficacious than anandamide as a CB" transporter, the anandamide amidohydrolase (fatty acid amide agonist, as assessed by measuring the stimulatory effect on intra- hydrolase, FAAH) and the two cannabinoid receptor subtypes, cellular Ca#+ mobilization in undifferentiated N18TG2 neuro- CB" and CB#. NADAs competitively inhibited FAAH from blastoma cells. This effect of AA-DA was counteracted by the l µ N18TG2 cells (IC&! 19–100 M), as well as the binding of the CB" antagonist SR141716A. -

The Cannabinoid Receptor Agonist WIN 55,212-2 Attenuates the Effects Induced by Quinolinic Acid in the Rat Striatum
Neuropharmacology 51 (2006) 1004e1012 www.elsevier.com/locate/neuropharm The cannabinoid receptor agonist WIN 55,212-2 attenuates the effects induced by quinolinic acid in the rat striatum A. Pintor a, M.T. Tebano a, A. Martire a, R. Grieco a, M. Galluzzo a, M.L. Scattoni b,A.Pe`zzola a, R. Coccurello c, F. Felici a, V. Cuomo d, D. Piomelli e, G. Calamandrei b, P. Popoli a,* a Department of Drug Research and Evaluation, Central Nervous System Pharmacology Division, Istituto Superiore di Sanita`, Viale Regina Elena, 299, 00161 Rome, Italy b Department of Cell Biology and Neuroscience, Istituto Superiore di Sanita`, Viale Regina Elena, 299, 00161 Rome, Italy c Institute of Neuroscience, EBRI Foundation, Rome, Italy d Department of Pharmacology and General Physiology, University ‘‘La Sapienza’’, Rome, Italy e Department of Pharmacology and Center for Drug Discovery, University of California, Irvine, CA, USA Received 7 April 2006; received in revised form 15 May 2006; accepted 16 June 2006 Abstract The ability of CB1 receptors to regulate the release of glutamate in the striatum, together with the finding that, in experimental models of Huntington disease (HD), both endocannabinoid levels and CB1 receptor densities are reduced, has prompted the investigation on the neuropro- tective role of the cannabinoids in HD. Quinolinic acid (QA) is an excitotoxin that, when injected in the rat striatum reproduces many features of HD and that acts by stimulating glutamate outflow. The aim of the present study was to test the ability of the cannabinoid receptor agonist WIN 55,212-2 to prevent the effects induced by QA in the rat striatum. -

Potential Cannabis Antagonists for Marijuana Intoxication
Central Journal of Pharmacology & Clinical Toxicology Bringing Excellence in Open Access Review Article *Corresponding author Matthew Kagan, M.D., Cedars-Sinai Medical Center, 8730 Alden Drive, Los Angeles, CA 90048, USA, Tel: 310- Potential Cannabis Antagonists 423-3465; Fax: 310.423.8397; Email: Matthew.Kagan@ cshs.org Submitted: 11 October 2018 for Marijuana Intoxication Accepted: 23 October 2018 William W. Ishak, Jonathan Dang, Steven Clevenger, Shaina Published: 25 October 2018 Ganjian, Samantha Cohen, and Matthew Kagan* ISSN: 2333-7079 Cedars-Sinai Medical Center, USA Copyright © 2018 Kagan et al. Abstract OPEN ACCESS Keywords Cannabis use is on the rise leading to the need to address the medical, psychosocial, • Cannabis and economic effects of cannabis intoxication. While effective agents have not yet been • Cannabinoids implemented for the treatment of acute marijuana intoxication, a number of compounds • Antagonist continue to hold promise for treatment of cannabinoid intoxication. Potential therapeutic • Marijuana agents are reviewed with advantages and side effects. Three agents appear to merit • Intoxication further inquiry; most notably Cannabidiol with some evidence of antipsychotic activity • THC and in addition Virodhamine and Tetrahydrocannabivarin with a similar mixed receptor profile. Given the results of this research, continued development of agents acting on cannabinoid receptors with and without peripheral selectivity may lead to an effective treatment for acute cannabinoid intoxication. Much work still remains to develop strategies that will interrupt and reverse the effects of acute marijuana intoxication. ABBREVIATIONS Therapeutic uses of cannabis include chronic pain, loss of appetite, spasticity, and chemotherapy-associated nausea and CBD: Cannabidiol; CBG: Cannabigerol; THCV: vomiting [8]. Recreational cannabis use is on the rise with more Tetrahydrocannabivarin; THC: Tetrahydrocannabinol states approving its use and it is viewed as no different from INTRODUCTION recreational use of alcohol or tobacco [9]. -

Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back
International Journal of Molecular Sciences Review Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back Osnat Almogi-Hazan * and Reuven Or Laboratory of Immunotherapy and Bone Marrow Transplantation, Hadassah Medical Center, The Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 91120, Israel; [email protected] * Correspondence: [email protected] Received: 21 May 2020; Accepted: 19 June 2020; Published: 23 June 2020 Abstract: The Cannabis plant contains numerous components, including cannabinoids and other active molecules. The phyto-cannabinoid activity is mediated by the endocannabinoid system. Cannabinoids affect the nervous system and play significant roles in the regulation of the immune system. While Cannabis is not yet registered as a drug, the potential of cannabinoid-based medicines for the treatment of various conditions has led many countries to authorize their clinical use. However, the data from basic and medical research dedicated to medical Cannabis is currently limited. A variety of pathological conditions involve dysregulation of the immune system. For example, in cancer, immune surveillance and cancer immuno-editing result in immune tolerance. On the other hand, in autoimmune diseases increased immune activity causes tissue damage. Immuno-modulating therapies can regulate the immune system and therefore the immune-regulatory properties of cannabinoids, suggest their use in the therapy of immune related disorders. In this contemporary review, we discuss the roles of the endocannabinoid system in immunity and explore the emerging data about the effects of cannabinoids on the immune response in different pathologies. In addition, we discuss the complexities of using cannabinoid-based treatments in each of these conditions. -

The Cannabinoid WIN 55,212-2 Prevents Neuroendocrine Differentiation of Lncap Prostate Cancer Cells
OPEN Prostate Cancer and Prostatic Diseases (2016) 19, 248–257 www.nature.com/pcan ORIGINAL ARTICLE The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells C Morell1, A Bort1, D Vara2, A Ramos-Torres1, N Rodríguez-Henche1 and I Díaz-Laviada1 BACKGROUND: Neuroendocrine (NE) differentiation represents a common feature of prostate cancer and is associated with accelerated disease progression and poor clinical outcome. Nowadays, there is no treatment for this aggressive form of prostate cancer. The aim of this study was to determine the influence of the cannabinoid WIN 55,212-2 (WIN, a non-selective cannabinoid CB1 and CB2 receptor agonist) on the NE differentiation of prostate cancer cells. METHODS: NE differentiation of prostate cancer LNCaP cells was induced by serum deprivation or by incubation with interleukin-6, for 6 days. Levels of NE markers and signaling proteins were determined by western blotting. Levels of cannabinoid receptors were determined by quantitative PCR. The involvement of signaling cascades was investigated by pharmacological inhibition and small interfering RNA. RESULTS: The differentiated LNCaP cells exhibited neurite outgrowth, and increased the expression of the typical NE markers neuron-specific enolase and βIII tubulin (βIII Tub). Treatment with 3 μM WIN inhibited NK differentiation of LNCaP cells. The cannabinoid WIN downregulated the PI3K/Akt/mTOR signaling pathway, resulting in NE differentiation inhibition. In addition, an activation of AMP-activated protein kinase (AMPK) was observed in WIN-treated cells, which correlated with a decrease in the NE markers expression. Our results also show that during NE differentiation the expression of cannabinoid receptors CB1 and CB2 dramatically decreases. -

The Determination of CBD and General Cannabinoid Content In
No. SSI-HPLC-018 High Performance Liquid Chromatography The Determination of CBD and General Cannabinoid Content in Hemp Oils Using HPLC with UV Detection No. HPLC-018 ■ Introduction Medical marijuana generally possesses high levels of CBD oil is derived as concentrate from CO2 or the therapeutic cannabidiol, CBD, and lower levels butane extraction of hemp, sometimes followed by (generally less than 0.3%) of the psychotropic steam distillation or ethanol distillation for tetrahydrocannabinol, d9-THC. Pain mitigation and purification. The Farm Bill of 2014 distinguishes reduced severity of nausea and seizures are just a hemp from marijuana, yet interpreting the law is few of the therapeutic benefits reported by medical difficult in that “CBD oil” may be classified as cannabis patients. Little has been done to better marijuana. understand the chemistry of benefits from CBD. To complicate matters, there is evidence that a The FDA has issued warning letters to firms that combination of CBD, a host of other minor market unapproved new drugs allegedly contain cannabinoids and a complex array of terpenoids may CBD. As part of these actions, the FDA has be the most beneficial – called the “entourage determined the cannabinoid content of some hemp effect.” CBD-rich oil has become increasingly products and many were found to contain levels of popular and is administered via sublingual drops, gel CBD that are very different from the label claim. It is capsules or as a topical ointment. important to note that such products are not approved by the FDA for the diagnosis, cure, The main source of CBD-rich oil is industrial hemp. -

SNP in Potentially Defunct Tetrahydrocannabinolic Acid Synthase Is a Marker for Cannabigerolic Acid Dominance in Cannabis Sativa L
G C A T T A C G G C A T genes Article SNP in Potentially Defunct Tetrahydrocannabinolic Acid Synthase Is a Marker for Cannabigerolic Acid Dominance in Cannabis sativa L. Andrea R. Garfinkel * , Matthew Otten and Seth Crawford Oregon CBD, Independence, OR 97351, USA; [email protected] (M.O.); [email protected] (S.C.) * Correspondence: [email protected] Abstract: The regulation of cannabinoid synthesis in Cannabis sativa is of increasing research interest as restrictions around the globe loosen to allow the plant’s legal cultivation. Of the major cannabi- noids, the regulation of cannabigerolic acid (CBGA) production is the least understood. The purpose of this study was to elucidate the inheritance of CBGA dominance in C. sativa and describe a marker related to this chemotype. We produced two crossing populations, one between a CBGA dominant cultivar and a tetrahydrocannabinolic acid (THCA) dominant cultivar, and one between a CBGA dominant cultivar and a cannabidiolic acid (CBDA) cultivar. Chemical and genotyping analyses confirmed that CBGA dominance is inherited as a single recessive gene, potentially governed by a non-functioning allelic variant of the THCA synthase. The “null” THCAS synthase contains a single nucleotide polymorphism (SNP) that may render the synthase unable to convert CBGA to THCA leading to the accumulation of CBGA. This SNP can be reliably used as a molecular marker for CBGA dominance in the selection and breeding of C. sativa. Citation: Garfinkel, A.R.; Otten, M.; Keywords: cannabinoids; cannabinoid synthesis; CBG; hemp Crawford, S. SNP in Potentially Defunct Tetrahydrocannabinolic Acid Synthase Is a Marker for Cannabigerolic Acid Dominance in 1. -

Cannabidiol and ( ) 9-Tetrahydrocannabinol Are Neuroprotective Antioxidants
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8268–8273, July 1998 Medical Sciences Cannabidiol and (2)D9-tetrahydrocannabinol are neuroprotective antioxidants A. J. HAMPSON*†,M.GRIMALDI‡,J.AXELROD*, AND D. WINK§ *Laboratory of Cellular and Molecular Regulation, National Institutes of Mental Health, Bethesda, MD 20892; ‡Laboratory of Adaptive Systems, National Institute of Neurological Disorders and Stroke, Bethesda, MD 20892; and §Radiology and Biology Branch, National Cancer Institute, Bethesda, MD 20892 Contributed by Julius Axelrod, April 27, 1998 ABSTRACT The neuroprotective actions of cannabidiol reduce the toxic reactive oxygen species (ROS) formed during and other cannabinoids were examined in rat cortical neuron ischemic metabolism. Cannabinoids like (2)D9-tetrahydrocan- cultures exposed to toxic levels of the excitatory neurotrans- nabinol (THC) and its psychoactive analogues also have been mitter glutamate. Glutamate toxicity was reduced by both reported to be neuroprotective against glutamate toxicity in cannabidiol, a nonpsychoactive constituent of marijuana, and vitro (7). Cannabinoids have been suggested to prevent gluta- the psychotropic cannabinoid (2)D9-tetrahydrocannabinol mate neurotoxicity by activating cannabinoid receptors (7, 8), (THC). Cannabinoids protected equally well against neuro- which can reduce calcium influx through voltage sensitive toxicity mediated by N-methyl-D-aspartate receptors, 2-ami- calcium channels (8, 9). A synthetic cannabinoid (HU-211) no-3-(4-butyl-3-hydroxyisoxazol-5-yl)propionic acid recep- also has been demonstrated to be neuroprotective even though tors, or kainate receptors. N-methyl-D-aspartate receptor- it does not activate cannabinoid receptors. This compound is induced toxicity has been shown to be calcium dependent; this an atypical cannabinoid, however, in that it, unlike other study demonstrates that 2-amino-3-(4-butyl-3-hydroxyisox- cannabinoids, directly antagonizes NMDAr (10) and possesses azol-5-yl)propionic acidykainate receptor-type neurotoxicity some antioxidant properties (11). -

Cannabis Withdrawal and Sleep: a Systematic Review of Human Studies
Sleep Medicine Reviews 18 (2014) 477e487 Contents lists available at ScienceDirect Sleep Medicine Reviews journal homepage: www.elsevier.com/locate/smrv CLINICAL REVIEW The effects of cannabinoid administration on sleep: a systematic review of human studies Peter J. Gates*, Lucy Albertella, Jan Copeland National Cannabis Prevention and Information Centre, UNSW Medicine, Australia article info summary Article history: This paper reviews the literature regarding the effects of cannabinoid administration on sleep in humans. Received 2 December 2013 A literature search using a set of cannabinoid and sleep-related terms was conducted across eight Received in revised form electronic databases. Human studies that involved the administration of cannabinoids and at least one 27 February 2014 quantitative sleep-related measure were included. Review papers, opinion pieces, letters or editorials, Accepted 27 February 2014 case studies (final N < 7), published abstracts, posters, and non-English papers were excluded. Thirty- Available online 7 March 2014 nine publications were included in the review. Findings were mixed and showed various effects of cannabinoid administration on several aspects of sleep. Methodological issues in the majority of studies Keywords: fi Cannabis to date, however, preclude any de nitive conclusion. Ó Marijuana 2014 Elsevier Ltd. All rights reserved. Sleep Insomnia Introduction understood motive is use to assist with sleep problems. This motive is not uncommon and has been reported by one quarter of a large Cannabis is the most frequently used illicit drug around the sample of cannabis using high school graduates [9]. Indeed, globe and is estimated to be used by approximately 4.5% of the intoxication from cannabis use is most commonly described to world’s population e a prevalence which is currently increasing [1]. -
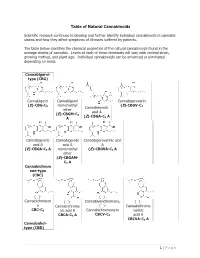
Table of Natural Cannabinoids
Table of Natural Cannabinoids Scientific research continues to develop and further identify individual cannabinoids in cannabis strains and how they affect symptoms of illnesses suffered by patients. The table below identifies the chemical properties of the natural cannabinoids found in the average strains of cannabis. Levels of each of these chemicals will vary with varietal strain, growing method, and plant age. Individual cannabinoids can be enhanced or eliminated depending on need. Cannabigerol- type (CBG) Cannabigerol Cannabigerol Cannabigerovarin (E)-CBG-C monomethyl (E)-CBGV-C 5 Cannabinerolic 3 ether acid A (E)-CBGM-C 5 (Z)-CBGA-C A A 5 Cannabigerolic Cannabigerolic Cannabigerovarinic acid acid A acid A A (E)-CBGA-C5 A monomethyl (E)-CBGVA-C3 A ether (E)-CBGAM- C5 A Cannabichrom ene-type (CBC) (±)- (±)- Cannabichromen (±)- Cannabivarichromene, (±)- e Cannabichrome (±)- Cannabichrome CBC-C5 nic acid A Cannabichromevarin varinic CBCA-C5 A CBCV-C3 acid A CBCVA-C3 A Cannabidiol- type (CBD) 1 | Page (−)-Cannabidiol Cannabidiol Cannabidiol-C4 (−)- Cannabidiorc CBD-C5 momomethyl CBD-C4 Cannabidivarin ol ether CBDV-C3 CBD-C1 CBDM-C5 Cannabidiolic Cannabidivarini acid c acid CBDA-C5 CBDVA-C3 Cannabinodiol- type (CBND) Cannabinodiol Cannabinodivar CBND-C5 in CBND-C3 Tetrahydrocan nabinol-type (THC) 9 9 9 Δ - Δ - Δ - Δ9- Tetrahydrocanna Tetrahydrocan Tetrahydrocannabivarin 9 Tetrahydrocan binol nabinol-C4 Δ -THCV-C3 9 9 nabiorcol Δ -THC-C5 Δ -THC-C4 9 Δ -THCO-C1 9 9 Δ -Tetrahydro- Δ9-Tetrahydro- Δ -Tetrahydro- Δ9-Tetrahydro- cannabinolic -

Acetaminophen Relieves Inflammatory Pain Through CB1 Cannabinoid Receptors in the Rostral Ventromedial Medulla
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Acetaminophen Relieves Inflammatory Pain Through CB1 Cannabinoid Receptors in the Rostral Ventromedial Medulla Pascal P. Klinger-Gratz1, William T. Ralvenius1, Elena Neumann1, Ako Kato2, Rita Nyilas2, Zsolt Lele2, István Katona2 and Hanns Ulrich Zeilhofer1,3 1Institute of Pharmacology and Toxicology, University of Zurich, Winterthurerstrasse 190, CH-8057, Switzerland 2Institute of Experimental Medicine, Hungarian Academy of Sciences, 43 Szigony Street, H-1083 Budapest, Hungary 3Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology (ETH) Zurich, Vladimir-Prelog-Weg 1-5/10, CH-8093 Zürich, Switzerland DOI: 10.1523/JNEUROSCI.1945-17.2017 Received: 9 July 2017 Revised: 27 October 2017 Accepted: 14 November 2017 Published: 22 November 2017 Author contributions: P.P.K.-G., W.T.R., E.N., A.K., R.N., Z.L., and I.K. performed research; P.P.K.-G., W.T.R., E.N., A.K., R.N., Z.L., I.K., and H.U.Z. analyzed data; W.T.R., I.K., and H.U.Z. designed research; I.K. and H.U.Z. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. The authors thank Drs. Beat Lutz and Giovanni Marsicano for providing CB1fl/fl mice, Dr. Masahiko Watanabe for the CB1 receptor antibody, Sébastien Druart, Andreas Pospischil, Roseline Weilenmann for the analyses of biomarkers of liver damage, and Isabelle Kellenberger, Balázs Pintér, Erika Tischler and Louis Scheurer for technical assistance. The work was partially supported by a grant from Federal Government of Switzerland through the Swiss Contribution (SH7/2/18) to IK and HUZ and by the Hungarian Academy of Sciences Momentum Program LP-54/2013 (to IK).