(G-Csfs) (Filgrastim, Lenograstim, Lipegfilgrastim and Pegfilgrastim): New Warning Regarding Aortitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Class Overview Colony Stimulating Factors
Therapeutic Class Overview Colony Stimulating Factors Therapeutic Class Overview/Summary: This review will focus on the granulocyte colony stimulating factors (G-CSFs) and granulocyte- macrophage colony stimulating factors (GM-CSFs).1-5 Colony-stimulating factors (CSFs) fall under the naturally occurring glycoprotein cytokines, one of the main groups of immunomodulators.6 In general, these proteins are vital to the proliferation and differentiation of hematopoietic progenitor cells.6-8 The G- CSFs commercially available in the United States include pegfilgrastim (Neulasta®), filgrastim (Neupogen®), filgrastim-sndz (Zarxio®), and tbo-filgrastim (Granix®). While filgrastim-sndz and tbo- filgrastim are the same recombinant human G-CSF as filgrastim, only filgrastim-sndz is considered a biosimilar drug as it was approved through the biosimilar pathway. At the time tbo-filgrastim was approved, a regulatory pathway for biosimilar drugs had not yet been established in the United States and tbo-filgrastim was filed under its own Biologic License Application.9 Only one GM-CSF is currently available, sargramostim (Leukine). These agents are Food and Drug Administration (FDA)-approved for a variety of conditions relating to neutropenia or for the collection of hematopoietic progenitor cells for collection by leukapheresis.1-5 Due to the pathway taken, tbo-filgrastim does not share all of the same indications as filgrastim and these two products are not interchangeable. It is important to note that although filgrastim-sndz is a biosimilar product, and it was approved with all the same indications as filgrastim at the time, filgrastim has since received FDA-approval for an additional indication that filgrastim-sndz does not have, to increase survival in patients with acute exposure to myelosuppressive doses of radiation.1-3A complete list of indications for each agent can be found in Table 1. -

FULPHILA Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FULPHILA safely and effectively. See full prescribing information for -----------------------WARNINGS AND PRECAUTIONS---------------------- FULPHILA. • Fatal splenic rupture: Evaluate patients who report left upper abdominal or shoulder pain for an enlarged spleen or splenic rupture. (5.1) ™ FULPHILA (pegfilgrastim-jmdb) injection, for subcutaneous use • Acute respiratory distress syndrome (ARDS): Evaluate patients who Initial U.S. Approval: 2018 develop fever, lung infiltrates, or respiratory distress. Discontinue ™ ® Fulphila in patients with ARDS. (5.2) FULPHILA (pegfilgrastim-jmdb) is biosimilar* to NEULASTA • Serious allergic reactions, including anaphylaxis: Permanently (pegfilgrastim). (1) discontinue Fulphila in patients with serious allergic reactions. (5.3) • Fatal sickle cell crises: Have occurred. (5.4) ----------------------------INDICATIONS AND USAGE--------------------------- • Glomerulonephritis: Evaluate and consider dose-reduction or Fulphila is a leukocyte growth factor indicated to interruption of Fulphila if causality is likely. (5.5) • Decrease the incidence of infection, as manifested by febrile neutropenia, in patients with non-myeloid malignancies receiving ------------------------------ADVERSE REACTIONS------------------------------ myelosuppressive anti-cancer drugs associated with a clinically Most common adverse reactions (≥ 5% difference in incidence compared to significant incidence of febrile neutropenia. -

2018 Annual Report on Eudravigilance for the European Parliament, the Council and the Commission Reporting Period: 1 January to 31 December 2018
21 March 2019 EMA/906394/2019 Inspections, Human Medicines Pharmacovigilance and Committees Division 2018 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission Reporting period: 1 January to 31 December 2018 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. Reproduction is authorised provided the source is acknowledged. Table of contents Abbreviations used in the document ...................................................................................... 3 1. Executive summary ............................................................................................................ 4 2. Operation of EudraVigilance including its new functionalities ............................................ 6 3. Data collection and data quality ......................................................................................... 7 Medicinal product information ..................................................................................................... 7 Reporting of ADR reports and patient involvement ........................................................................ 7 Data Quality ............................................................................................................................. 8 4. Data analysis .................................................................................................................... -

Comparison Between Filgrastim and Lenograstim Plus Chemotherapy For
Bone Marrow Transplantation (2010) 45, 277–281 & 2010 Macmillan Publishers Limited All rights reserved 0268-3369/10 $32.00 www.nature.com/bmt ORIGINAL ARTICLE Comparison between filgrastim and lenograstim plus chemotherapy for mobilization of PBPCs R Ria, T Gasparre, G Mangialardi, A Bruno, G Iodice, A Vacca and F Dammacco Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine and Clinical Oncology, University of Bari Medical School, Bari, Italy Recombinant human (rHu) G-CSF has been widely used during transplantation.4 However, the use of G-CSF after to treat neutropenia and mobilize PBPCs for their autologous PBPC transplantation has been queried, as its autologous and allogeneic transplantation. It shortens further reduction in time to a safe neutrophil count5,6 does neutropenia and thus reduces the frequency of neutropenic not always imply fewer significant clinical events, such as fever. We compared the efficiency of glycosylated rHu infections, length of hospitalization, extrahematological and non-glycosylated Hu G-CSF in mobilizing hemato- toxicities or mortality.7,8 Even so, the ASCO guidelines still poietic progenitor cells (HPCs). In total, 86 patients were recommend the use of growth factors after autologous consecutively enrolled for mobilization with CY plus either PBPC transplantation.9 glycosylated or non-glycosylated G-CSF, and under- G-CSF induces the proliferation and differentiation of went leukapheresis. The HPC content of each collection, myeloid precursor cells, and also provides a functional toxicity, days of leukapheresis needed to reach the activity that influences chemotaxis, respiratory burst and minimum HPC target and days to recover WBC (X500 Ag expression of neutrophils. -

Filgrastim Vs Pegfilgrastim: a Quality of Life Issue for Children
FEATURE | Filgrastim vs pegfilgrastim Filgrastim vs pegfilgrastim: A quality of life issue for children Administration, safety, and efficacy are similar in both agents.H owever, the frequency of administration makes a significant difference for patients. KAREN E. MACDonaLD, BSN, RN, CPON; HAYLEY BEE, BSN, RN, CPN, CCRN; DARBY TOZER, BSN, RN; JEnnIFER E. TRAIN, BSN, RN ancer is diagnosed in 1.5 million people in the United States each year, and more than 12,000 cancer patients are younger C 1 than 21 years. Parents sit across the table from the medical team and learn about the side effects of treatment that their child may experience. The child will lose his or her hair, miss school, experience nausea and vomiting, and endure multiple laboratory and diagnostic tests. Families learn that their day-to-day life, once filled with school, work, soccer games, and other family- centered activities, will now consist of hospital admissions, doctor visits, and isolation to abate the possible side effects of treatment. In addition, parents will need to learn how to administer clinical care, such as subcutaneous injections of medications to improve the child’s immune system, at home. Neutrophils are a critical member of the phago- cytic system and provide a first-line defense against bacterial organisms.2 Neutropenia is defined as a reduction in circulating neutrophils to less than 1,500/µL.1 Chemotherapy-induced neutropenia is the primary treatment-related dose-limiting toxicity in children with can- cer. Severe neutropenia (neutrophils less than 500/µL) can occur as a result of chemotherapy treatment. Children who receive intensive che- motherapy have a 40% chance of developing 3 © THINKSTOCK © febrile neutropenia. -

G-CSF Protects Motoneurons Against Axotomy-Induced Apoptotic Death In
Henriques et al. BMC Neuroscience 2010, 11:25 http://www.biomedcentral.com/1471-2202/11/25 RESEARCH ARTICLE Open Access G-CSF protects motoneurons against axotomy- induced apoptotic death in neonatal mice Alexandre Henriques1,2,3, Claudia Pitzer1*, Luc Dupuis2,3, Armin Schneider1* Abstract Background: Granulocyte colony stimulating factor (G-CSF) is a growth factor essential for generation of neutrophilic granulocytes. Apart from this hematopoietic function, we have recently uncovered potent neuroprotective and regenerative properties of G-CSF in the central nervous system (CNS). The G-CSF receptor and G-CSF itself are expressed in a motoneurons, G-CSF protects motoneurons, and improves outcome in the SOD1(G93A) transgenic mouse model for amyotrophic lateral sclerosis (ALS). In vitro, G-CSF acts anti- apoptotically on motoneuronal cells. Due to the pleiotrophic effects of G-CSF and the complexity of the SOD1 transgenic ALS models it was however not possible to clearly distinguish between directly mediated anti- apoptotic and indirectly protective effects on motoneurons. Here we studied whether G-CSF is able to protect motoneurons from purely apoptotic cell death induced by a monocausal paradigm, neonatal sciatic nerve axotomy. Results: We performed sciatic nerve axotomy in neonatal mice overexpressing G-CSF in the CNS and found that G-CSF transgenic mice displayed significantly higher numbers of surviving lumbar motoneurons 4 days following axotomy than their littermate controls. Also, surviving motoneurons in G-CSF overexpressing animals were larger, suggesting additional trophic effects of this growth factor. Conclusions: In this model of pure apoptotic cell death the protective effects of G-CSF indicate direct actions of G-CSF on motoneurons in vivo. -

The Impact of Biosimilar Competition in Europe December 2020
White Paper The Impact of Biosimilar Competition in Europe December 2020 PER TROEIN, Vice President, Strategic Partners, IQVIA MAX NEWTON, Senior Consultant, Global Supplier & Association Relations, IQVIA KIRSTIE SCOTT, Analyst, Global Supplier & Association Relations, IQVIA Table of contents Introduction 1 Key observations 2 Methodology 11 Country and therapy area KPIs 14 Human growth hormone (HGH) 14 Epoetin (EPO) 16 Granulocyte-colony stimulating factor (GCSF) 18 Anti-tumour necrosis factor (ANTI-TNF) 20 Fertility (FOLLITROPIN ALFA) 22 Insulins 24 Oncology 26 Low-molecular-weight heparin (LMWH) 28 Appendix 30 EMA list of approved biosimilars 30 List of Biosimilars under review by EMA 32 Introduction ‘The Impact of Biosimilar Competition in Europe’ report describes the effects on price, volume, and market share following the arrival of biosimilar competition in Europe. The report consists of: observations on competitive markets, and a set of Key Performance Indicators (KPIs) to monitor the impact of biosimilars in 23 European markets. The report has been a long-standing source of information on the status of the biosimilars market. This iteration has been delayed due to the COVID-19 pandemic across the globe and has provided an opportunity to provide full-year 2019 data, and an additional data point (June 2020 MAT) which incorporates the impact on patients in Europe across major therapeutic areas to 30th June 2020. The direct impact of which is visible in the Low Molecular Weight Heparin (LMWH), and Fertility (somatropin) markets. This report has been prepared by IQVIA at the The European Medicines Agency (EMA) has a central request of the European Commission services with role in setting the rules for biosimilar submissions, initial contributions on defining the KPIs from EFPIA, approving applications, establishing approved Medicines for Europe, and EuropaBio. -

©Ferrata Storti Foundation
Stem Cell Transplantation • Research Paper Single-dose pegfilgrastim for the mobilization of allogeneic CD34+ peripheral blood progenitor cells in healthy family and unrelated donors Frank Kroschinsky Background and Objectives. Short-term treatment with granulocyte colony-stimulating Kristina Hölig factor (G-CSF) has been established as the standard regimen for mobilizing allogeneic Kirsten Poppe-Thiede peripheral blood progenitor cells (PBPC) from healthy donors. The pegylated form of fil- Kristin Zimmer grastim (pegfilgrastim) has a longer elimination half-life because of decreased serum Rainer Ordemann clearance and might be a convenient alternative for stem cell mobilization. Matthias Blechschmidt Uta Oelschlaegel Design and Methods. Twenty-five family (n=15) or unrelated (n=10) healthy donors received a single-dose of 12 mg pegfilgrastim for mobilization of allogeneic PBPC. Martin Bornhauser + Gabi Rall Donors with inadequate mobilization (blood CD34 cells ≤5/µL on day 3 or ≤20/µL on Claudia Rutt day 4) were given additional daily doses of 10 µg/kg conventional filgrastim. Gerhard Ehninger Leukapheresis was planned to start on day 5. Results. All harvests were completed successfully. In 20 out of 25 donors (80 %) only a single apheresis was necessary. Additional non-pegylated filgrastim had to be given to only one 74-year old family donor. The maximum concentration of circulating CD34+ + cells occurred on day 5 (median 67/µL, range 10-385/µL). The median yield of CD34 cells was 9.3 (range 3.2-39.1) 106/kg of the recipient´s body weight. The median × 8 number of T cells in the apheresis products was 3.9 (range 2.7-10.8)×10 /kg. -

Human G-CSF ELISA Kit (ARG80143)
Product datasheet [email protected] ARG80143 Package: 96 wells Human G-CSF ELISA Kit Store at: 4°C Component Cat. No. Component Name Package Temp ARG80143-001 Antibody-coated 8 X 12 strips 4°C. Unused strips microplate should be sealed tightly in the air-tight pouch. ARG80143-002 Standard 3 X 2 ng/vial 4°C (Lyophilized) ARG80143-003 Standard diluent 20 ml 4°C buffer ARG80143-004 Antibody conjugate 400 µl 4°C concentrate ARG80143-005 Antibody diluent 16 ml 4°C buffer ARG80143-006 HRP-Streptavidin 400 µl 4°C (Protect from concentrate light) ARG80143-007 HRP-Streptavidin 16 ml 4°C diluent buffer ARG80143-008 20X Wash buffer 50 ml 4°C ARG80143-009 TMB substrate 12ml 4°C (Protect from light) ARG80143-010 STOP solution 12ml 4°C ARG80143-011 Plate sealer 4 strips Room temperature Summary Product Description ARG80143 Human G-CSF ELISA Kit is an Enzyme Immunoassay kit for the quantification of Human G-CSF in Serum, Plasma, Cell culture supernatants. Tested Reactivity Hu Tested Application ELISA Specificity No significant cross-reactivity or interference with Human CT-1,IL-11,OSM,CNTF,HGF,M-CSF;mouse IL-6;rat IL-6,CNTF Target Name G-CSF Conjugation HRP Conjugation Note Substrate: TMB and read at 450 nm Sensitivity 15 pg/ml Sample Type Serum, Plasma, Cell culture supernatants Standard Range 31.25 - 2000 pg/ml www.arigobio.com 1/3 Sample Volume 100 µl Precision CV: Alternate Names Granulocyte colony-stimulating factor; Lenograstim; C17orf33; GCSF; G-CSF; Filgrastim; Pluripoietin; CSF3OS Application Instructions Assay Time 4 hours Properties Form 96 well Storage instruction Store the kit at 2-8°C. -

NEULASTA (Pegfilgrastim)
HIGHLIGHTS OF PRESCRIBING INFORMATION Injection: 6 mg/0.6 mL solution in a single-dose prefilled syringe These highlights do not include all the information needed to use co-packaged with the on-body injector for Neulasta. (3) NEULASTA safely and effectively. See full prescribing information for NEULASTA. -------------------------------CONTRAINDICATIONS --------------------------- Patients with a history of serious allergic reactions to human granulocyte NEULASTA® (pegfilgrastim) injection, for subcutaneous use colony-stimulating factors such as pegfilgrastim or filgrastim. (4) Initial U.S. Approval: 2002 -----------------------WARNINGS AND PRECAUTIONS----------------------- ----------------------------RECENT MAJOR CHANGES------------------------- Fatal splenic rupture: Evaluate patients who report left upper abdominal Warnings and Precautions, Aortitis (5.11) 0 6 / 2 0 1 8 or shoulder pain for an enlarged spleen or splenic rupture. (5.1) W a r n i n g s a n d P r e c a u t i o n s , N u c l e a r I m a g i n g ( 5 . 1 2 ) 06/2018 Acute respiratory distress syndrome (ARDS): Evaluate patients who develop fever, lung infiltrates, or respiratory distress. Discontinue ----------------------------INDICATIONS AND USAGE--------------------------- Neulasta in patients with ARDS. (5.2) Neulasta is a leukocyte growth factor indicated to Serious allergic reactions, including anaphylaxis: Permanently Decrease the incidence of infection, as manifested by febrile discontinue Neulasta in patients with serious allergic reactions. (5.3) neutropenia, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically The on-body injector for Neulasta uses acrylic adhesive. For patients significant incidence of febrile neutropenia. (1.1) who have reactions to acrylic adhesives, use of this product may result in a significant reaction. -

2020, Vol. 16, Number 1, 1–40
Oncology in Clinical Practice 2020, Vol. 16, Number 1, 1–40 Oncology in Clinical Practice 2020, Vol. 2020, Vol. 16, Number 1 ISSN 2450–1654 Beata Jagielska, Elżbieta Sarnowska, Tomasz Sarnowski, Katarzyna Śmiałek-Kania, Janusz Siedlecki, Andrzej Kawecki Contemporary diagnostic and therapeutic possibilities in patients with adenoid cystic carcinoma of the head and neck Łukasz Kwinta Febrile neutropenia prophylaxis with short- and long-acting granulocyte colony-stimulating factors during treatment of solid tumours Bartłomiej Żerek, Wojciech Straś, Piotr Rózga, Elżbieta Pękala The evolution of biologics in the context of oncological therapy Aleksandra Sobiborowicz, Anna M Czarnecka, Anna Szumera-Ciećkiewicz, Piotr Rutkowski, Tomasz Świtaj Diagnosis and treatment of malignant PEComa tumours Monika Misztal, Magdalena Krakowska, Monika Ryś-Bednarska, Mariusz Śliwa, Piotr Potemski Symptoms of nervous system damage in a patient undergoing anti-PD1 immunotherapy Róża Poźniak-Balicka, Dawid Murawa Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) ONCOLOGY IN CLINICAL PRACTICE Official Journal of the Polish Society of Clinical Oncology https://journals.viamedica.pl/oncology_in_clinical_practice Editor-in-Chief dr hab. med. Maria Litwiniuk prof. dr hab. med. Maciej Krzakowski dr med. Aleksandra Łacko prof. Ruggero De Maria (Rome, Italy) Deputy Editors dr Mario Mandala (Bergamo, Italy) prof. dr hab. med. Andrzej Kawecki dr hab. med. Radosław Mądry prof. dr hab. med. Piotr Potemski dr med. Janusz Meder prof. dr hab. med. Piotr Rutkowski dr hab. med. Sergiusz Nawrocki prof. dr hab. med. Krzysztof Składowski prof. dr hab. med. Włodzimierz Olszewski prof. dr hab. med. Piotr Wysocki prof. dr hab. med. Maria Podolak-Dawidziak dr hab. med. Barbara Radecka Scientific Board prof. -
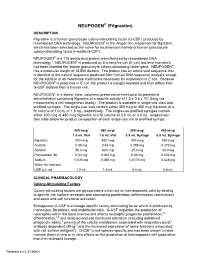
NEUPOGEN® (Filgrastim) DESCRIPTION Filgrastim Is a Human Granulocyte Colony-Stimulating Factor (G-CSF)‚ Produced by Recombinant DNA Technology
NEUPOGEN® (Filgrastim) DESCRIPTION Filgrastim is a human granulocyte colony-stimulating factor (G-CSF)‚ produced by recombinant DNA technology. NEUPOGEN® is the Amgen Inc. trademark for filgrastim‚ which has been selected as the name for recombinant methionyl human granulocyte colony-stimulating factor (r-metHuG-CSF). NEUPOGEN® is a 175 amino acid protein manufactured by recombinant DNA technology.1 NEUPOGEN® is produced by Escherichia coli (E coli) bacteria into which has been inserted the human granulocyte colony-stimulating factor gene. NEUPOGEN® has a molecular weight of 18‚800 daltons. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis‚ except for the addition of an N-terminal methionine necessary for expression in E coli. Because NEUPOGEN® is produced in E coli‚ the product is nonglycosylated and thus differs from G-CSF isolated from a human cell. NEUPOGEN is a sterile‚ clear‚ colorless‚ preservative-free liquid for parenteral administration containing filgrastim at a specific activity of 1.0 ± 0.6 x 108 U/mg (as measured by a cell mitogenesis assay). The product is available in single-use vials and prefilled syringes. The single-use vials contain either 300 mcg or 480 mcg filgrastim at a fill volume of 1.0 mL or 1.6 mL, respectively. The single-use prefilled syringes contain either 300 mcg or 480 mcg filgrastim at a fill volume of 0.5 mL or 0.8 mL, respectively. See table below for product composition of each single-use vial or prefilled syringe. 300 mcg/ 480 mcg/ 300 mcg/ 480 mcg/ 1.0 mL Vial 1.6 mL Vial 0.5 mL Syringe 0.8 mL Syringe filgrastim 300 mcg 480 mcg 300 mcg 480 mcg Acetate 0.59 mg 0.94 mg 0.295 mg 0.472 mg Sorbitol 50.0 mg 80.0 mg 25.0 mg 40.0 mg Polysorbate 80 0.04 mg 0.064 mg 0.02 mg 0.032 mg Sodium 0.035 mg 0.056 mg 0.0175 mg 0.028 mg Water for Injection USP q.s.