Review of Toxic Epidermal Necrolysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
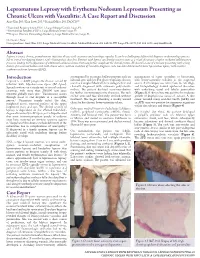
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Diagnosis, Classification, and Management of Erythema
Arch Dis Child 2000;83:347–352 347 Diagnosis, classification, and management of Arch Dis Child: first published as 10.1136/adc.83.4.347 on 1 October 2000. Downloaded from erythema multiforme and Stevens–Johnson syndrome C Léauté-Labrèze, T Lamireau, D Chawki, J Maleville, A Taïeb Abstract become widely accepted that EM and SJS, as Background—In adults, erythema multi- well as toxic epidermal necrolysis, are all part of forme (EM) is thought to be mainly a single “EM spectrum”. In both EM and SJS, related to herpes infection and Stevens– pathological changes in the earliest skin lesion Johnson syndrome (SJS) to drug reac- consist of the accumulation of mononuclear tions. cells around the superficial dermal blood Aims—To investigate this hypothesis in vessels; epidermal damage is more characteris- children, and to review our experience in tic of EM with keratinocyte necrosis leading to the management of these patients. multilocular intraepidermal blisters.5 In fact, Methods—A retrospective analysis of 77 there is little clinical resemblance between paediatric cases of EM or SJS admitted to typical EM and SJS, and recently some authors the Children’s Hospital in Bordeaux be- have proposed a reconsideration of the “spec- tween 1974 and 1998. trum” concept and a return to the original Results—Thirty five cases, inadequately description.15–17 According to these authors, the documented or misdiagnosed mostly as term EM should be restricted to acrally urticarias or non-EM drug reactions were distributed typical targets or raised oedema- excluded. Among the remaining 42 pa- tous papules. Depending on the presence or tients (14 girls and 28 boys), 22 had EM (11 absence of mucous membrane erosions the EM minor and 11 EM major), 17 had SJS, cases may be classified as EM major or EM 16 and three had isolated mucous membrane minor. -

Drug Eruptions
DRUG ERUPTIONS http://www.aocd.org A drug eruption is an adverse skin reaction to a drug. Many medications can cause reactions, especially antimicrobial agents, sulfa drugs, NSAIDs, chemotherapy agents, anticonvulsants, and psychotropic drugs. Drug eruptions can imitate a variety of other skin conditions and therefore should be considered in any patient taking medications or that has changed medications. The onset of drug eruptions is usually within 2 weeks of beginning a new drug or within days if it is due to re-exposure to a certain drug. Itching is the most common symptom. Drug eruptions occur in approximately 2-5% of hospitalized patients and in greater than 1% of the outpatient population. Adverse reactions to drugs are more prevalent in women, in the elderly, and in immunocompromised patients. Drug eruptions may be immunologically or non-immunologically mediated. There are 4 types of immunologically mediated reactions, with Type IV being the most common. Type I is immunoglobulin-E dependent and can result in anaphylaxis, angioedema, and urticaria. Type II is cytotoxic and can result in purpura. Type III reactions are immune complex reactions which can result in vasculitis and type IV is a delayed-type reaction which results in contact dermatitis and photoallergic reactions. This is important as different medications are associated with different types of reactions. For example, insulin is related with type I reactions whereas penicillin, cephalosporins, and sulfonamides cause type II reactions. Quinines and salicylates can cause type III reactions and topical medications such as neomycin can cause type IV reactions. The most common drugs that may potentially cause drug eruptions include amoxicillin, trimethoprim sulfamethoxazole, ampicillin, penicillin, cephalosporins, quinidine and gentamicin sulfate. -

Erythema Multiforme: Recognition and Management Kathryn P
Erythema Multiforme: Recognition and Management Kathryn P. Trayes, MD; Gillian Love, MD; and James S. Studdiford, MD Thomas Jefferson University Hospital, Philadelphia, Pennsylvania Erythema multiforme is an immune-mediated reaction that involves the skin and sometimes the mucosa. Classically described as target-like, the erythema multiforme lesions can be isolated, recur- rent, or persistent. Most commonly, the lesions of erythema multiforme present symmetrically on the extremities (especially on extensor surfaces) and spread centripetally. Infections, especially herpes simplex virus and Mycoplasma pneumoniae, and medications constitute most of the causes of erythema multiforme; immunizations and autoimmune diseases have also been linked to erythema multiforme. Erythema multiforme can be differentiated from urticaria by the duration of individual lesions. Ery- thema multiforme lesions are typically fixed for a minimum of seven days, whereas individual urticarial lesions often resolve within one day. Erythema multiforme can be confused with the more serious con- dition, Stevens-Johnson syndrome; however, Stevens-Johnson syndrome usually contains widespread erythematous or purpuric macules with blisters. The management of erythema multiforme involves symptomatic treatment with topical steroids or antihistamines and treating the underlying etiology, if known. Recurrent erythema multiforme associated with the herpes simplex virus should be treated with prophylactic antiviral therapy. Severe mucosal erythema multiforme can require hospitalization -

Drug Eruption
Drug eruption March 25,2015 Outline • Clinical features • Pathogenesis • How to approach? • Management? Need to know • Urticaria • Exanthematous rash • DRESS • Stevens-Johnson syndrome/TEN • Fix drug eruptions • Acute generalized exanthematous pustulosis • Photoallergic/Phototoxic. • Chemotherapy induced.. Generalized erythematous and slightly edematous maculopapular rashes Erythema and edema of face and periorbital area Investigations 28/8/47 30/8/47 2/9//47 Total 1110 1690 2024 Eosinophil SGOT 42 98 69 SGPT 130 108 88 Your Dx is D R E S S ? Drug Rash with Eosinophilia and Systemic Symptoms DRESS • Aromatic antiepileptic agents (phenytoin, carbamazepine, phenobarbital) • Sulfonamides, allopurinol, gold salts, dapsone, and minocycline. 5 days after prednisolone 30mg/d 5 days after prednisolone 30mg/d Gout after 2 weeks of allopurinol Toxic Epidermal Necrolysis from allopurinol Approach to the Acute Generalized Blistering Patient History : Onset ,underlying disease, New Drug ,other symptoms? ( fever,sore throat ) Physical examination : target lesion nikolsky sign, epidermal necrolysis, mucosal involvement Investigation : baseline lab,skin biopsy + Direct Immonofluorescence Differential diagnosis of TEN • SSSS (Staphylococcal scalded skin syndrome ) • Autoimmune blistering disease ( pemphigus,linear IgA dermatosis.. ) • Erythema multiforme Generalized exanthem Blistering ,denudation Generalized Cutaneous tenderness Nikolsky sign + desquamation Apoptosis desmoglein-1 TEN SSSS Pemphigus vulgaris Bullous pemphigoid Erythema multiforme Take -

Exanthems and Drug Reactions
Dermatology Exanthems and Morton Rawlin drug reactions ‘Well, Mr Jones, I think we should put you on this tablet to Background fix this problem. Now, the things you need to look out for Drug reactions are a common cause of rashes and can vary are any rashes…’ from brief, mildly annoying, self limiting rashes to severe conditions involving multiple organ systems. How often in general practice do you hear yourself Objective offering this advice? Why do almost all drugs list rash as This article outlines an approach to exanthems that a side effect? How do they occur and what can you do to may be related to drug reactions and details appropriate recognise and manage them? management. The skin is the largest organ of the body and, from a diagnostic Discussion viewpoint, we can see it change to various stimuli. Medications Rashes related to drug reactions are both nonallergic and allergic. Nonallergic rashes are usually predictable and are commonly used and are integral to the general practitioner’s may be avoidable. Allergic rashes include morbilliform armamentarium for treating most ills. However, it is also important erythema, urticaria and angioedema, erythema multiforme to note that increasing access to medications by consumers through and vasculitic rashes. The vast majority of cases are other health professionals (eg. naturopaths) and the self prescribed rapidly resolving and self limiting once the offending use of over-the-counter, complementary and alternative medicines agent is removed. Early recognition and supportive should be remembered in the history taking of a patient presenting measures are the keys to care in the majority of cases. -

Asboe-Hansen Sign in Toxic Epidermal Necrolysis
CASE LETTER Asboe-Hansen Sign in Toxic Epidermal Necrolysis Jessica R. Dowling, BA; Kathryn L. Anderson, MD; William W. Huang, MD, MPH immunofluorescence, which was negative for IgG, C3, PRACTICE POINTS IgM, and IgA. Based on the clinical presentation involving • Asboe-Hansen sign is a useful clinical tool for diag- more than 30% of the patient’s body surface area (BSA) nosing toxic epidermal necrolysis (TEN). and the pathology findings, a diagnosis of toxic epider- • Asboe-Hansen sign can be employed to generate mal necrolysis (TEN) was made. The patient remained a fresh bulla for lesional skin biopsy in the evaluation in the intensive care unit with a multidisciplinary team of TEN. consisting of dermatology, ophthalmology, gynecology, gastroenterology,copy and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the To the Editor: patient made a full recovery. A 25-year-old woman with no notable medical history Toxic epidermal necrolysis is a rare, acute, life- was admitted to the hospital for suspected Stevens- threateningnot mucocutaneous disease within a spectrum of Johnson syndrome (SJS). The patient was started on adverse cutaneous drug reactions. The estimated world- amoxicillin 7 days prior to the skin eruption for prophy- wide incidence of TEN is 0.4 to 1.9 per million individuals laxis before removal of an intrauterine device. On theDo day annually. 1 Toxic epidermal necrolysis is clinically charac- of admission, she reported ocular discomfort, dysphagia, terized by diffuse exfoliation of the skin and mucosae with and dysuria. She developed erythema of the conjunctivae, flaccid bullae. -

Impetigo in Children: a Clinical Guide and Treatment Options
Review: Impetigo in children: a clinical guide and treatment options Impetigo in children: a clinical guide and treatment options Motswaledi MH, MBChB, MMed(Derm), FCDerm (SA) Department of Dermatology, University of Limpopo, Medunsa Campus Correspondence to: Dr MH Motswaledi, e-mail: [email protected] Keywords: impetigo, impetigo contagiosa, bullous impetigo, children, skin infection Abstract Impetigo is a contagious, superficial bacterial infection of the skin, most frequently encountered in children. Causative organisms are almost always Staphylococcus aureus or streptococci, or a combination of the two. Predisposing factors are nasal and perineal colonisation, overcrowding, poor personal hygiene, minor skin trauma and pre-existing skin diseases with disrupted skin barrier function, like eczema. Infection is mainly acquired through contact with sufferers or nasal carriers. Treatment should be given to avoid spread of the disease, and to minimise the risk of infecting others. Although the majority of cases of impetigo are self-limiting, under certain circumstances complications like toxic shock syndrome, staphylococcal osteomyelitis, septic arthritis and pneumonia can occur. Furthermore, certain strains of group A β-haemolytic streptococci causing impetigo may result in poststreptococcal glomerulonephritis, just like streptococcal throat infections can result in rheumatic fever in children, but the pathogenesis remains poorly understood. It appears to be due to abnormal immune response or hypersensitivity to streptococcal antigens. S Afr Fam Pract 2011;53(1):44-46 Introduction The vesicles rupture rapidly, and as a result, they are seldom seen. The exuding serum dries to form brownish Impetigo is a common bacterial skin infection in children. crusts with a characteristic honey colour (Figure 1). -

Blanching Rashes
BLANCHING RASHES Facilitators Guide Author Aoife Fox (Edits by the DFTB Team) [email protected] Author Aoife Fox Duration 1-2h Facilitator level Senior trainee/ANP and above Learner level Junior trainee/Staff nurse and Senior trainee/ANP Equipment required None OUTLINE ● Pre-reading for learners ● Basics ● Case 1: Chicken Pox (15 min) ● Case 2: Roseola (15 min) ● Case 3: Scarlet fever (20 min) ● Case 4: Kawasaki disease (including advanced discussion) (25 min) ● Game ● Quiz ● 5 take home learning points PRE-READING FOR LEARNERS BMJ Best Practice - Evaluation of rash in children PEDS Cases - Viral Rashes in Children RCEM Learning - Common Childhood Exanthems American Academy of Dermatology - Viral exanthems 2 Infectious Non-infectious Blanching Blanching Staphylococcus scalded skin syndrome Sunburn Impetigo Eczema Bullous impetigo Urticaria Eczema hepeticum Atopic dermatitis Measles Acne vulgaris Glandular fever/infectious mononucleosis Ichthyosis vulgaris keratosis pilaris Hand foot and mouth disease Salmon patch Erythema infectiosum/Fifth disease Melasma Chickenpox (varicella zoster) Napkin rash Scabies Seborrhoea Tinea corporis Epidermolysis bullosa Tinea capitis Kawasaki disease Molluscum contagiosum Steven-Johnson syndrome Scarlet fever Steven-Johnson syndrome/toxic epi- Lyme disease dermal necrolysis Congenital syphilis Erythema multiforme Congenital rubella Erythema nodosum Herpes simplex Roseola (sixth disease) Non-blanching Epstein-Barr virus Port-wine stain Pityriasis rosea Henoch-Schoenlein purpura Idiopathic thrombocytopenia Acute leukaemia Haemolytic uremic syndrome Trauma Non-blanching Mechanical (e.g. coughing, vomiting – in Meningococcal rash distribution of superior vena cava) 3 BASE Key learning points Image: used with gratitude from Wikipedia.org Definitions/rash description: ● Macule: a flat area of colour change <1 cm in size (e.g., viral exanthem [such as measles and rubella], morbilliform drug eruption). -

Impetigo/Staphylococcal Scalded Skin Disease Lorena C
Briefin Impetigo/Staphylococcal Scalded Skin Disease Lorena C. Dollani, MD,*†‡ Kalyani S. Marathe, MD* *Children’s National Medical Center, Washington, DC †Washington Hospital Center, Washington, DC ‡Georgetown University Hospital, Washington, DC Bacterial skin infections are among the most common skin diseases in children. These encompass a range of cutaneous manifestations from localized (bullous impetigo) to systemic (staphylococcal scalded skin disease [SSSS]). The most common pathogen in both nonbullous and bullous impetigo is Staphylococcus aureus. Another important pathogen causing nonbullous impetigo is group A b- hemolytic Streptococcus. SSSS specifically refers to a spectrum of skin diseases induced by the exfoliative toxins of S aureus. The most common pathogen implicated in their pathophysiology is S aureus, which is a gram-positive coccus and can commonly colonize the nose, perineum, eyes, axillae, umbilicus, and wound sites. Impetigo frequently involves children younger than 6 years, accounting for approximately 10% of skin problems observed in pediatric clinics. SSSS is a rare blistering manifestation affecting mainly neonates and young children. Impetigo is an extremely contagious infection that can spread quickly via direct person-to- person contact or through fomites, and its peak incidence is in the summer AUTHOR DISCLOSURE Drs Dollani and months. Primary impetigo can result from direct bacterial involvement of the Marathe have disclosed no financial previously normal skin, whereas secondary impetigo is caused by a disruption in relationships relevant to this article. This commentary does not contain a discussion the skin barrier that allows the bacteria to adhere, invade, and establish an of an unapproved/investigative use of a infection. Causes of secondary impetigo include minor skin trauma secondary to commercial product/device. -

Varicella and Herpes Zoster Vaccines
INFORMATION SHEET OBSERVED RATE OF VACCINE REACTIONS Global Vaccine Safety, Immunization, Vaccines and Biologicals VARICELLA ZOSTER VIRUS VACCINE 20, avenue Appia, Ch-1211 Geneva 27 June 2012 The Vaccines Monovalent varicella vaccine The varicella-zoster virus (VZV) vaccine to protect against varicella (varicella vaccine) is composed of the Oka strain of live attenuated virus. The Oka strain was isolated in Japan from a healthy child with natural varicella, and was attenuated through sequential passages in cultures of human embryonic lung cells, embryonic guinea-pig cells and human diploid cell line WI-38. The virus underwent further passages through human diploid cell line MCR-5 for one of the available vaccines. The vaccine is presented as lyophilized virus. Its medium is also supplied for reconstitution immediately before injection. Each 0.5 ml vaccine dose also contains 12.5 mg of hydrolyzed gelatine or human albumin, 25 mg of sucrose or lactose, trace amounts of neomycin and fetal bovine serum, and traces of residual components of substrate cell cultures (including DNA and protein), all within allowed range (see table below). The vaccine does not contain any preservative (CDC, 1996). Combination varicella vaccine Introduction of varicella vaccination for public health use in young children would be facilitated if varicella vaccine could be combined with a measles–mumps–rubella (MMR) vaccine. The titre of VZV is ~14 times higher in MMRV vaccine than in the monovalent varicella vaccine. The vaccine has been proven safe, with the exception -

Erythema Necroticans: a Presenting Manifestation of Silent Leprosy
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Journal of the Saudi Society of Dermatology & Dermatologic Surgery (2011) 15,63–66 King Saud University Journal of the Saudi Society of Dermatology & Dermatologic Surgery www.ksu.edu.sa www.jssdds.org www.sciencedirect.com CASE REPORT Erythema necroticans: A presenting manifestation of silent leprosy Nazeeha Al Hayki *, Badria Al-Mahmoud Dermatology & Venereology Department, Hamad Medical Corporation, P.O. Box 3050, Doha, Qatar Received 25 February 2011; accepted 30 March 2011 Available online 2 June 2011 KEYWORDS Abstract Leprosy reactions are rare expression of immunological perturbations that interrupt the Leprosy; usual chronic course and the clinical stability of patients with leprosy. Erythema nodosum leprosum Erythema nodosum (ENL) is an immune complex-mediated reaction that may complicate the course of multibacillary leprosum; leprosy. It generally occurs during antimycobacterial treatment and characterized by the appear- Erythema necroticans; ance of crops of brightly erythematous tender nodules or plaques. Severe ENL can become vesicular Multibacillary MDT or bollous and break-down and is termed erythema necroticans [Jobling, W.H., Mc Dougall, A.C., 1996. Leprosy reactions. In: Handbook of leprosy, 5th ed. CBS Publishers, New Delhi, pp. 82–91]. We present here a case of erythema necroticans, misdiagnosed as sweet’s syndrome, because he had never been presented with pre-existing evidence of leprosy nor had any antimycobacterial treat- ment. The clinical diagnosis is confirmed by microscopic pathology. The lesions resolved completely following multibacillary MDT, corticosteroids and Azathioprine. ª 2011 King Saud University. Production and hosting by Elsevier B.V.