Electrode Position and the Clinical Outcome After Bilateral Subthalamic Nucleus Stimulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interpreting the CBCT Data Volume Part I
MilesCE_Supp_Layout 1 7/8/11 12:59 PM Page FC1 Educational Supplement 2 ADA CERP CE Credits Orthotown is pleased to offer you continuing education. You can read the following CE article, take the post-test and claim your CE credits. See instructions on page 6. This CE course is supported by an unre- Farran Media, LLC, is an ADA CERP Recognized provider. ADA CERP is a service of the American Dental Association to assist dental professionals in identifying quality providers of continuing dental education. ADA CERP does not approve or endorse stricted grant from Cybermed, Inc. This individual courses or instructors, nor does it imply acceptance of credit hours by boards of dentistry. course offers two ADA CERP CE Credits. For more information, please visit http://www.towniecentral.com/orthotown/onlineCE.aspx MilesCE_Supp_Layout 1 7/7/11 12:26 PM Page 1 Part 1: Interpreting the CBCT Data Volume in Orthodontic Cases: You Should See What You May Be Missing! by Dale A. Miles, DDS, MS, FRCD(C) This is a two-part article designed to help clinicians The Skull and Its Contents understand the more common findings they will encounter in Most commonly there are physiologic calcifications seen the anatomic regions they capture in larger field-of-view within the skull on large field-of-view CBCT machines. (FOV) CBCT machines. Many of these findings will also be These include the more common calcifications of the pineal seen in smaller FOV machines when the volume capture is gland, choroid plexuses and falx cerebri. Although calcifica- moved around to view things like the temporomandibular tion of the falx cerebri is seen in the nevoid basal cell carci- joint or third molar regions. -

Comparison of Two Software Programs in Voxel-Based Superimposition of CBCT Images
@2019 JCO, Inc. May not be distributed without permission. www.jco-online.com Comparison of Two Software Programs in Voxel-Based Superimposition of CBCT Images TAREK ELSHEBINY, BDS, MSD SHERIF ELBARNASHAWY, BDS EHAB BENNASIR, BDS, MSD MOHAMED ADEL NADIM, BDS, MSD, PhD JUAN MARTIN PALOMO, DDS, MSD hile introducing the cephalometer in 1931, Broadbent proposed a tech- nique for superimposing successive cephalometric films as a way to Wstudy the physical changes caused by facial growth over time.1 This approach eventually became a standard component of orthodontic records. Since Broadbent, various authors have developed other methods of super- imposing serial cephalograms in the horizontal and vertical dimensions.2,3 An overall superimposition is usually performed on the cranial base to eval- uate growth and treatment outcomes by indicating changes in the maxillary and mandibular arches; regional superimpositions of the maxilla or mandible show dentoalveolar changes. A third dimension has always been lacking The resolution of a CBCT image is deter- in traditional cephalometric analysis and superim- mined by the individual volume elements (voxels) position, even though its importance in orthodon- produced from the volumetric dataset.5 A voxel is tic and surgical planning has been emphasized for the three-dimensional equivalent of the pixel in decades. Several attempts have been made to vi- two-dimensional images. The size of a voxel is sualize this third dimension,3-5 but it was the ad- defined by its height, width, and depth; the voxels vent of cone-beam computed tomography (CBCT) in CBCT scans are usually isotropic.6 The mono- and related software that enabled the assessment chromatic gray color of a voxel represents its den- of all three dimensions in clinical practice. -

Periodontal Measurement on Digital Images Reveals Risk of Overestimation in Clinical Periodontal Probing in Periodontitis Patients
Periodontal Measurement on Digital Images Reveals Risk of Overestimation in Clinical Periodontal Probing in Periodontitis Patients Hye-Min Chung Yonsei University College of Dentistry Jin-Young Park Yonsei University College of Dentistry Kyung-A Ko Yonsei University College of Dentistry Seong-Ho Choi Yonsei University College of Dentistry Jung-Seok Lee ( [email protected] ) Yonsei University College of Dentistry Research Article Keywords: Periodontitis, gingivitis, imaging, diagnosis Posted Date: June 30th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-654031/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/11 Abstract Aim: To compare the supra-alveolar gingival dimension (GD) and the clinical pocket probing depth (PD) by combining data from an intraoral scanner (IOS) and cone-beam computed tomography (CBCT), and identify the clinical features affecting the clinical PD. Materials and Methods: 1,071 sites from 11 patients were selected for whom CBCT, IOS images, and periodontal charts were recorded at the same visit. CBCT and IOS data were superimposed. GD was measured on cross-sectional images of the probed sites. The level of agreement and correlation between GD and PD were assessed for the entire population and also within groups (treated vs untreated, bleeding on probing [BOP] vs no BOP, and PDs of 0–3 mm vs 4–5 mm vs ≥6 mm). Results: The difference between GD and PD was 0.82±0.69 mm (mean±standard deviation), and they were positively correlated (r=0.790, p<0.001). The mean difference was smaller for sites with PD ≥4 mm than for those with a PD of 0–3 mm. -

The Effect of Amniotic Chorion Membrane on Tissue Biotype, Wound Healing and Periodontal Regeneration
IOSR Journal of Dental and Medical Sciences (IOSR - JDMS) e - ISSN: 2279 - 0853, p - ISSN: 2279 - 0861.Volume 17, Issue 12 Ver. 4 (December. 2018), PP 61 - 69 www.iosrjournals.org The Effect of Amniotic Chorion Membrane on Tissue Biotype, Wound Healing and Periodontal Regeneration Maha R. Taala b 1 , Raneem M. Gamal 2 1,2 Oral Medicine, Periodontology, Oral Diagnosis and Radiology Department, Faculty of Dentistry, Alexandria University, Alexandria, Egypt. Corresponding Author: Maha R. Taalab A bstract : Amniotic chorion membrane is considered to be a promising treatment modalities for furcation involvement . The aim of this study was to evaluate clinically and radiographically the effect of using Amniotic chorion membrane (ACM) in conjunction to alloplast bone graft in the management of grade II furcation involvement and to assess its effect on tissue biotype an d healing at the furcation area. This study was conducted on fourteen patients with fourteen furcation defects grade II. Patients were divided into two equal groups: Test group: were treated by alloplast bone graft and amnion chorion membrane, and control group: were treated with alloplast bone graft and collagen membrane. Soft tissue examination and hard tissue measurements with cone - beam computed tomography were performed. Test group has shown better healing, more gain in clinical attachment loss, impro ve tissue biotype and enhanced bone formation when compared to the control group. It was concluded that, ACM can promote periodontal regeneration, improve tissue biotype and enhance healing of periodontal wounds. ------------------------------------------- -------------------------------------------------------------------------- ------------- ----- Date of Submission: 27 - 11 - 2018 Date of accep tance: 10 - 12 - 2018 -------------------------- --------------------------------------------------------------------------------------------- ------------- --- I. Introduction Tissue biotype is a critical factor that determines the result of dental treatment. -

Tca Ballot 06 REVISED.Qxd
Ballot Anesthesiology LumaCool Whitening System & LumaWhite Plus – LumaLite, Inc. LUMIBrite Chairside Whitening – Den-Mat Holdings LLC Anesthetics – Local (Injected) Meta – Remedent Inc. Astra Zeneca Niveous – Shofu Dental Corporation COOK-WAITE – Carestream Dental Opalescence Boost – Ultradent Products, Inc. Septocaine – Septodont Perfect Bleach Office – VOCO America, Inc. Xylocaine, Polocaine, Citanest – DENTSPLY Professional Perfection White – Premier Dental Pola Office Plus – SDI (North America) Inc. Anesthetics – Technique System ProphyWhite – Life-Like Cosmetic Solutions Comfort Control – DENTSPLY Professional Rapid Rx In Office Teeth Whitening – Nu Radiance, Inc. DentalVibe Injection Comfort System – DentalVibe Rembrandt Products – Johnson & Johnson Healthcare Products Onset – Precision Buffering of Local Anesthetics – Onpharma Inc. Venus White Max – Heraeus STA System – Milestone Scientific Visible White Chairside – Colgate-Palmolive Co. X-Tip Intraosseus Anesthetic Delivery System – ZOOM! – Philips Consumer Lifestyle DENTSPLY Maillefer Cosmetic Bleaching – Take-Home system Anesthetics – Topicals AcquaBrite Nightime – Acquamed Technologies, Inc. Behzocaine Oral Anesthetic Gel – DENTSPLY Professional Dr. Collins All White Bleaching System – CaineTips – J. Morita USA, Inc. Dr. Collins Dental Products Denti-Care Denti-Freeze Topical Anesthetic Gel – Medicom Fluorescent Whitening System – Vista Dental Products EMLA – Astra Zeneca GC TiON – GC America Inc. GumNumb – Crosstex International KoR Whitening Deep Bleaching System – Hurricane -

The Use of Cone-Beam Computed Tomography in Furcation Defects Diagnosis
10.1515/bjdm-2016-0023 Y T E I C O S L BALKAN JOURNAL OF DENTAL MEDICINE A ISSN 2335-0245 IC G LO TO STOMA The Use of Cone-Beam Computed Tomography in Furcation Defects Diagnosis SUMMARY M. Cimbaljevic, J. Misic, S. Jankovic, Background: The use of cone-beam computed tomography (CBCT), as N. Nikolic-Jakoba an additional diagnostic tool in daily dental practice, has expanded rapidly School of Dental Medicine in recent years. Since CBCT allows assessment of dento-maxillofacial Department of Periodontology structures in three-dimensional manner, its use may be very tempting in University of Belgrade alveolar bone furcation defects (FDs) diagnosis. Belgrade, Serbia Aim: The aim of this study was to determine the impact of clinical experience and experience with CBCT on FD detection in patients with periodontitis. Material and Methods: Fifteen patients with chronic generalized severe periodontitis were included in the study. In total, 168 furcation sites were analyzed on CBCT images by a previously trained senior year undergraduate student (O1) and a PhD student with three years of CBCT experience (O2), and compared to clinical findings (probing). CBCT images were analyzed on two separate occasions, within a 7-day interval. FDs were assessed both clinically and on CBCT images, using a dichotomous scale (present/absent). Intraobserver agreement for each observer was calculated by using Kappa coefficient (k). Interobserver agreement and agreement between CBCT and clinical findings for both observers were calculated. Results: Kappa coefficient value for both observers indicated a high intraobserver agreement (k1=0.75; k2=0.94). Interobserver agreement of CBCT image analyses was present in 72.6% (73.0% in maxilla, 71.7% in mandible). -

NOV 2 3 2005 H 3
NOV 2 3 2005 h 3 510(k) Submission - Vimplant 510(k) Summary This summary of 51 0(k) safety and effectiveness information is being submitted in accordance with requirements of 21 CFR Part 807.92. Date: Aua 29. 2005 1. Company and Correspondent making the submission: Name - CyberMed, Inc. Address - #504 SJ Technoville- Gasan-dong 60-19, Geumcheon-gu, Seoul, 153-710, Korea Telephone - +82-2-3397-3970 Fax - +82-2-3397-3971 Contact - Mr. Song Nak Choi/ Manager Internet - http://www.cybermed.co.kr 2. Device: Trade/proprietary name :Vimplant Common Name :Dental implant simulation software Classification Name :Picture archiving and communications system 3. Predicate Devices : Manufacturer :MATERIALISE N.V. Device :SimPlant System 510(k) Number :K033849 (Decision Date - 05/25/2004) Manufacturer: CyberMed, Inc. Device: V-workSTM 510(k) Number: K01 3878 (Decision Date - 12/07/2001) 4. Classifications Names & Citations: 21CFR 820.2050, LLZ, Picture archiving and communications system, Class2 5. Description: 1) General Description Cyber~ed, Inc. 01)011(12 510O(k) Submission - Vimplant Vlmplant TM is a dental implant simulation software for dentists and implantologists. By use of VlmplantTm, dental practicians can plan and practice their surgery in advance so they can reduce some risks which can happen during their real surgery. Vlmplan tTM provides very useful and needful functions. it contains functions of implant simulation, manipulation with 2D and 3D mode, nerve identification, and evaluation of bone density. By using Vimplant TM, dental practicians can quickly and easily simulate implant surgery on their desktop PCs. This enables them to conduct implant surgery more effectively by isolating the exact implant position site and angle and to assist in deciding the proper implant diameter, length, etc. -

Understanding Digital Dentistry: Cbct and Intra
UNDERSTANDINGDIGITAL DENTISTRY: CBCT AND INTRA-ORAL 30 SCANNING -=- & UNDERSTANDINGDIGITAL DENTISTRY: CBCT AND INTRA-ORAL 30 SCANNING ----CBCTi-------iTERO------ NewTom VGi DIGITAL MODELS SYSTEM The iTero Experience *Vertical Patient Positioning *No More Goop, Gagging, or Discomfort *Medical Grade Tube Head *No tray and putty experience * Rotating Anode *Less mess more accuracy *.3mm Focal Spot *Faster more detailed digital models *Flat Panel *30 high quality imaging for better case *Safe Beam™ Technology (reduces planning radiation based on patient size) *Scan time: I 8sec Digital Models *X-Ray time: 5.2sec As iTero scans utilize I I-micron surface-tooth the leading 30 CBCT on the market, recognition software to create a highly NewTom is setting the industry standard accurate digital model of the patient's for patient experience and safety, image anatomical structure. quality, and 30 case diagnosis. Contact Points Choose the right implant software The iTero software allows doctors to examine the contact points of a patient's for your 30 case planning occlusion. Once a virtual wax-up is made contact points can be examined again to facilitate the fabrication of a provisional or a final crown. iTero Scan and Merge to CBCT iTero Scan CBCT Scan Doctors can perform implant placement using 30 imaging when the patient meets the specifications for an accurate merge of the iTero and CBCT scans. Your imaging center is responsible for all workflow leading up to treatment planning. Specifications for an Accurate Merge with CBCT: * At least 4 teeth on same arch *Pre-molar and molar on same side that each have an opposing tooth *Without these specifications an iTero scan will not work due to Jack of a virtual articulator and therefore vertical height cannot be established. -

Combining Virtual Model and Cone Beam Computed Tomography To
Kim et al. BMC Oral Health (2018) 18:180 https://doi.org/10.1186/s12903-018-0635-y RESEARCH ARTICLE Open Access Combining virtual model and cone beam computed tomography to assess periodontal changes after anterior tooth movement Sun-Hyun Kim1, Jong-Bin Lee2, Min-Ji Kim3 and Eun-Kyoung Pang4* Abstract Background: Orthodontic force may affect not only periodontal ligaments, but also the alveoloar bone and the gingiva according to the type of tooth movements. The authors assessed changes in gingival thickness (GT) and alveolar bone thickness (ABT) after orthodontic treatment using a new method. Methods: This study included 408 teeth (208 central incisors, 200 lateral incisors) from the upper and lower 4 anterior teeth of 52 patients who had completed orthodontic treatment. GT and ABT were measured using virtual casts fabricated from impressions and cone beam computed tomography (CBCT). Two sectioned images of every tooth axis were acquired by partitioning each tooth with a line connecting the midpoint of the incisal edge to the midpoint of the cementoenamel junction in the virtual models and the root apex in CBCT images. After superimposing the two sectioned images, GT and ABT were measured before and after orthodontic tooth movement. Correlations between GT and ABT before and after treatment, and changes in GT and ABT associated with sex, tooth arch, tooth position, orthognathic surgery, and tooth inclination and rotation were assessed. Results: Before orthodontic treatment, GT and ABT were significantly correlated. Patients who underwent orthognathic surgery exhibited an increase in GT thickness compared with those who did not. ABT was significantly decreased in proclined teeth and in rotated teeth. -
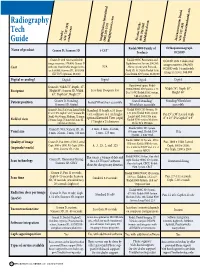
Radiography Tech Guide
Radiography Tech Soredex PracticeWorks Instrumentarium Dental Imaging Sciences International (414) 747-1030 (800) 944-6365 (414) 747-1030 (800) 205-3570 soredex.com kodakdental.com instrumentariumdental.com Guide imagingsciences.com Kodak 9000 Family of Orthopantomograph Name of product Cranex D; Scanora 3D i-CAT® Products OC200D Cranex D with two transferable Kodak 9000C Panoramic and OC200D with 2 dedicated image receivers, $70,400; Cranex D Cephalometric System, $90,545; N/A image receivers, $94,900; Cost with one transferable image receiv- Above system with Focused- OC200D with 1 transferable er, $59,900; Scanora 3D , $169,900 Field 3D, $129,000; Kodak 9500 (XV FOV Optional, $8,500) Cone Beam 3D System, $180,000 image receiver, $88,900 Digital or analog? Digital Digital Digital Digital Operational space: Kodak Cranex D, Width 72”, Depth, 47”, Width 74”; Depth 50”; Height 90”; Scanora 3D, Width Less than 16 square feet 9000C/9000C 3D Systems, 5.23 Footprint D x 7.0 W; Kodak 9500 System, Height 89” 63”, Depth 55”, Height 77” 5.66 D x 5.36 W Patient position Cranex D, Standing; Seated/Wheelchair accessible Seated/Standing/ Standing/Wheelchair Scanora 3D, Seated Wheelchair accessible accessible Cranex D, Pan 15 x 30 cm, Lateral Ceph Standard 13 height x 16 diam- Kodak 9000C 3D System, 50 8” x 10”, PA Ceph 8” x 10”; Scanora 3D, eter, collimate to 4 cm height; x 37 mm; Kodak 9500 system Pan 15” x 30”; Lateral Ceph Small, 60 x 60 mm; Medium, 75 mm x optional Extended View (ceph) Large Field, 184 x 206 mm; 8” x 10”; PA Ceph 8” x 9” Field of -

Radiology Article
radiology article by Thomas Giacobbi, DDS, FAGD Editorial Director, Dentaltown Magazine The future is now. Next month, at the American Dental Association (ADA) Annual Meeting in San Francisco, Education in the Round (EIR) will debut as the future of interactive dental education. A recent ADA press release describes it as such: “EIR is an interactive, multi- media, learning environment that supplements the need for workshops.” The room will serve as a forum for real-time demonstrations that will be projected on eight 61-inch flat screen monitors arranged in a central hub (See bottom of page). One EIR course, titled “Into the Third Dimension: The Present Future of Dental Care,” assured me that our timing for this particular product feature is right on target. The course description reads, “Live patient demonstrations using four of the latest 3-D imaging machines, including the i-CAT from Imaging Sciences International, Galileos by Sirona, NewTom VG by NewTom Dental, and the Iluma from IMTEC Imaging.” There are more than 10 cone beam CT (or cone beam volumetric tomography) machines on the market today. When you see that many $150,000-$250,000 machines in the dental market, it’s time to take notice. While the price tag is initially a bit of a shock, you must understand the potential of this technology and soon you might add it to your holiday wish list. Virtually every practicing dentist can benefit from this technology and you do not ADA Codes need to own a machine to benefit. In some communities there are imaging centers that D0360 Cone Beam CT – Craniofacial Data Capture can scan your patient and send the data electronically for your use in office. -
X a Focused Evaluation of Single-Unit Plasma Transfusions in a Tertiary
Focused Evaluation of Single-Unit Plasma Transfusions 0 in a Tertiary Care Academic Medical Center x AU SAINT LOUIS Thomas Fay, M.D., Daniela Hermelin, M.D., Douglas Blackall, M.D., M.RH. PAT H 0 LOGY uNIVERSITY. DEPARTMENT Department of Pathology, Saint Louis University School of Medicine SMWT LOI iVNPYIflJfl INTRODUCTION 1 STUDY DESIGN a Blood utilization monitoring isa component of our institutional PBM program. Data for 2017 plasma transfusions were At Saint Louis University Hospital (SLUH), an academic, tertiary care facility, we extracted from our electronic health perform concurrent daily blood utilization reviews. record system During our audit, a surprising number of single-unit plasma transfusions were Inclusion Criteria identified in patients with normal coagulation values ‘All patients were adults (>18 years old) This study was performed to evaluate single-unit plasma transfusions in detail, Patients further subdivided into two groups; to identify opportunities to improve transfusion practice. patients with INR values s1.5 and patients with INR values >1.5 ‘Patients with INR values 1.5 were the RESULTS primary focus of this study •Additional study data collected included the transfusing clinical service, a post-transfusion . A total of 2,887 units of plasma were transfused in 2017. INR value obtained within 24 hours of criteria. 395(14%) were single-unit transfusions; 328(11%) met the inclusion transfusion (if available), and the probable 55 single-unit transfusions were associated with INR values 1.5. indication for transfusion S Surgical subspecialties and emergency medicine were the most common Exclusion Criteria: transfusing services. ‘Patients transfused with plasma in the A post-transfusion INR, when available, did not demonstrate a significant operating room or post-anesthesia care unit change from the pre-transfusion baseline.