Relatively High-Protein Or 'Low-Carb'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein What It Is Protein Is Found in Foods from Both Plants and Animals
Protein What It Is Protein is found in foods from both plants and animals. Protein is made up of hundreds or thousands of smaller units, called amino acids, which are linked to one another in long chains. The sequence of amino acids determines each protein’s unique structure and its specific function. There are 20 different amino acids that that can be combined to make every type of protein in the body. These amino acids fall into two categories: • Essential amino acids are required for normal body functioning, but they cannot be made by the body and must be obtained from food. Of the 20 amino acids, 9 are considered essential. • Nonessential amino acids can be made by the body from essential amino acids consumed in food or in the normal breakdown of body proteins. Of the 20 amino acids, 11 are considered nonessential. Where It Is Found Protein is found in a variety of foods, including: • Beans and peas • Dairy products (such as milk, cheese, and yogurt) • Eggs • Meats and poultry • Nuts and seeds • Seafood (fish and shellfish) • Soy products • Whole grains and vegetables (these generally provide less protein than is found in other sources) What It Does • Protein provides calories, or “energy” for the body. Each gram of protein provides 4 calories. • Protein is a component of every cell in the human body and is necessary for proper growth and development, especially during childhood, adolescence, and pregnancy. • Protein helps your body build and repair cells and body tissue. • Protein is a major part of your skin, hair, nails, muscle, bone, and internal organs. -

Introduction to Proteins and Amino Acids Introduction
Introduction to Proteins and Amino Acids Introduction • Twenty percent of the human body is made up of proteins. Proteins are the large, complex molecules that are critical for normal functioning of cells. • They are essential for the structure, function, and regulation of the body’s tissues and organs. • Proteins are made up of smaller units called amino acids, which are building blocks of proteins. They are attached to one another by peptide bonds forming a long chain of proteins. Amino acid structure and its classification • An amino acid contains both a carboxylic group and an amino group. Amino acids that have an amino group bonded directly to the alpha-carbon are referred to as alpha amino acids. • Every alpha amino acid has a carbon atom, called an alpha carbon, Cα ; bonded to a carboxylic acid, –COOH group; an amino, –NH2 group; a hydrogen atom; and an R group that is unique for every amino acid. Classification of amino acids • There are 20 amino acids. Based on the nature of their ‘R’ group, they are classified based on their polarity as: Classification based on essentiality: Essential amino acids are the amino acids which you need through your diet because your body cannot make them. Whereas non essential amino acids are the amino acids which are not an essential part of your diet because they can be synthesized by your body. Essential Non essential Histidine Alanine Isoleucine Arginine Leucine Aspargine Methionine Aspartate Phenyl alanine Cystine Threonine Glutamic acid Tryptophan Glycine Valine Ornithine Proline Serine Tyrosine Peptide bonds • Amino acids are linked together by ‘amide groups’ called peptide bonds. -

Nutritional Value of Soybean Meal Produced from High Protein, Low Oligosaccharide, Or Conventional Varieties of Soybeans And
Animal Feed Science and Technology 188 (2014) 64–73 Contents lists available at ScienceDirect Animal Feed Science and Technology journal homepage: www.elsevier.com/locate/anifeedsci Nutritional value of soybean meal produced from high protein, low oligosaccharide, or conventional varieties of soybeans and fed to weanling pigs ∗ K.M. Baker, Y. Liu, H.H. Stein Department of Animal Sciences, University of Illinois, Urbana, USA a r a t i c l e i n f o b s t r a c t Article history: Three experiments were conducted to evaluate the nutritional value of high protein soybean Received 19 August 2013 meal (SBM-HP), low oligosaccharide soybean meal (SBM-LO), and conventional soybean Received in revised form 22 October 2013 meal (SBM-CV) fed to weanling pigs. The three soybean meals (SBM) contained 549, 536 Accepted 25 October 2013 and 475 g/kg crude protein, respectively. In Exp. 1, the coefficient of ileal standardized digestibility (CISD) of amino acids (AA) in the 3 ingredients was measured using eight bar- rows (initial body weight: 14.3 ± 1.23 kg) that were equipped with a T-cannula in the distal Keywords: ileum and allotted to a replicated 4×4 Latin square design with four periods (seven days Amino acids Energy per period) and four diets per square. Three diets contained SBM-HP, SBM-LO, or SBM-CV High protein as the sole source of AA. The fourth diet was a N-free diet that was used to determine basal Low oligosaccharide ileal endogenous losses of AA. Results indicated that the CISD for all AA was not different Soybean meal among the three varieties of SBM. -

Breeding for Quality Protein Maize (QPM) Varieties: a Review
agronomy Review Breeding for Quality Protein Maize (QPM) Varieties: A Review Liliane N. Tandzi 1,2,*, Charles S. Mutengwa 1, Eddy L. M. Ngonkeu 2,3, Noé Woïn 2 and Vernon Gracen 4 1 Department of Agronomy, Faculty of Science and Agriculture, University of Fort Hare, P. Bag X1314, Alice 5700, South Africa; [email protected] 2 Institute of Agricultural Research for Development (IRAD), P.O. Box 2123, Messa, Yaounde, Cameroon; [email protected] (E.L.M.N.); [email protected] (N.W.) 3 Department of Plant Biology and Physiology, Faculty of Science, University of Yaounde I, Yaounde, Cameroon 4 West Africa Centre for Crop Improvement (WACCI), College of Basic and Applied Sciences, University of Ghana, Legon PMB LG 30, Accra 999064, Ghana; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +27-063-459-4323 Received: 28 August 2017; Accepted: 19 October 2017; Published: 28 November 2017 Abstract: The nutritional evaluation of quality protein maize (QPM) in feeding trials has proved its nutritional superiority over non-QPM varieties for human and livestock consumption. The present paper reviews some of the most recent achievements in development of QPM varieties using both conventional and molecular breeding under stressed and non-stressed environments. It is evident that numerous QPM varieties have been developed and released around the world over the past few decades. While the review points out some gaps in information or research efforts, challenges associated with adoption QPM varieties are highlighted and suggestions to overcome them are presented. The adoption of released varieties and challenges facing QPM production at the farmer level are also mentioned. -

High Protein High Energy Food Choices
Oncology Nutrition High Protein High Energy Food Choices At times during treatment you may not feel like eating much or it may be difficult to eat. If you are losing weight, you may need to choose high protein, high energy foods. High protein foods help maintain and repair muscles and tissues that have been damaged during treatment. High energy foods provide the extra calories you need to help maintain your weight. This booklet provides ideas on how to include high protein high energy foods when your appetite is low. 2 High Protein High Energy Food Choices Tips to Help with a Poor Appetite • Eat your biggest meal when your appetite is best. This may be at breakfast time rather than at the evening meal. • Eat often through the day. • Try to eat something every 1 – 2 hours. • Have small snacks between meals everyday. Snacks can give you extra energy. See page 6 for snack ideas. • Include a high protein food at each meal and snack. See page 3 for protein foods. • Carry snacks with you if you are planning to be away from home • Try a few mouthfuls even if you are not hungry. • Eat high energy and high protein foods most of the time. Limit low fat, calorie reduced or diet foods. See page 3 for list of high protein and energy foods. • Drink liquids that give you energy through the day such as milk, smooothies, commercial nutritional supplement drinks or juice. See pages 7-8 for suggestions. Limit low calorie fluids such as water, coffee and tea as they will fill you up and not allow you to eat much at mealtimes. -

The Role of Amino Acids in Liver Protein Metabolism Under a High Protein Diet
The role of amino acids in liver protein metabolism under a high protein diet : identification of amino acids signal and associated transduction pathways Nattida Chotechuang To cite this version: Nattida Chotechuang. The role of amino acids in liver protein metabolism under a high protein diet : identification of amino acids signal and associated transduction pathways. Food and Nutrition. AgroParisTech, 2010. English. NNT : 2010AGPT0026. pastel-00610998 HAL Id: pastel-00610998 https://pastel.archives-ouvertes.fr/pastel-00610998 Submitted on 25 Jul 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° /__/__/__/__/__/__/__/__/__/__/ T H E S I S submitted to obtain the degree of Doctor of Philosophy at L’Institut des Sciences et Industries du Vivant et de l’Environnement (AgroParisTech) Speciality: Nutrition Science Presented and defended in public by Nattida CHOTECHUANG on 22nd March 2010 THE ROLE OF AMINO ACIDS IN LIVER PROTEIN METABOLISM UNDER A HIGH PROTEIN DIET: IDENTIFICATION OF AMINO ACIDS SIGNAL AND ASSOCIATED TRANSDUCTION PATHWAYS Thesis director: Daniel TOMÉ Thesis co-director: Dalila AZZOUT-MARNICHE AgroParisTech, UMR914 Nutrition Physiology and Ingestive Behaviour, F-75005 Paris to the jury: Mr. -

Protein Serving Size Tells You What a Single Portion Is
READ FOOD LABELS If you need to limit to find the best choice for your diet USE HEALTHY TIPS to shop, plan, and prepare meals with less protein Serving Size tells you what a single portion is. For soups At home protein Servings Per Container • Use lower-protein foods such as rice and pasta to add For main dishes lists how many portions per bulk to a soup. • Use vegetables and and low protein foods as your container. • Use low-protein milk substitutes when making main dish; use lean meats or other high-value protein % Daily Value is based on cream soups. as your side dish. a 2,000 calorie daily diet. • Try kebabs. Use smaller pieces of meat with more What is protein? This number helps you know For sandwiches vegetables or fruits. Protein is a nutrient that helps build muscle, repair if a food is high or low in • Fill sandwiches with lettuce, alfalfa sprouts, tissue, and fight infection a nutrient, even if you eat cucumber, chopped celery, apple, parsley or water • Prepare dishes with small pieces of meat, such more than 2,000 calories. chestnuts. Use breads that are low in protein. as chicken, mixed in with rice or pasta. Chicken Why limit protein? • Use breads that are low in protein and that are more or shrimp with rice or ground meat with pasta For people with early kidney disease, eating less thickly sliced. Or try more flavorful breads (such as works well. protein may help to: sourdough or rye bread). • For casseroles, use smaller amounts of meat than the recipe calls for, and increase the starch (rice • prevent wastes from building up in the blood Protein is listed per serving. -
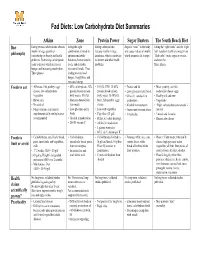
Fad Diets: Low Carbohydrate Diet Summaries
Fad Diets: Low Carbohydrate Diet Summaries Atkins Zone Protein Power Sugar Busters The South Beach Diet Diet Eating excess carbohydrates releases Eating the right Eating carbohydrates Sugar is “toxic” to the body Eating the “right carbs” and the “right insulin in large quantities combination of foods to releases insulin in large and causes release of insulin, fats” results in health and weight loss philosophy contributing to obesity and health optimize metabolic quantities, which contributes which promotes fat storage. “Bad carbs” create urges to overeat problems. Restricting carbohydrate functions, lowers insulin to obesity and other health and store fat leads to ketosis which decreases levels and desirable problems. Three phases hunger and increasing metabolism. eicosanoid levels. Thus Three phases leading to decreased hunger, weight loss, and increased energy. Foods to eat • All meats, fish, poultry, eggs, • 40% carbohydrate, 30% • 15-35% CHO, 30-45% • Protein and fat • Meat , poultry, and fish, cheese, low-carbohydrate protein (based on lean protein (based on lean • Low-glycemic index foods reduced fat cheese, eggs vegetables body mass), 30% fat body mass), 30-50% fat • Olive oil, canola oil in • Healthy oils and nuts • Butter, oils • Monounsaturated fats, • Meat, fish, poultry, eggs, moderation • Vegetables • No alcohol lean meats cheese • Alcohol in moderation • “Right carbohydrates and sweets”- • Mega vitamins and mineral • Low-glycemic-index • Low-carb vegetables • Fruits must be eaten alone. low GI supplements daily (multiple -

Sample Menu Plenty of Protein on a Vegetarian Or Vegan Diet
Plenty of Protein on a Sample Menu Vegetarian or Vegan Diet BREAKFAST A myth about plant-based diets is that it’s difficult to get enough 1 cup oatmeal (6g) protein. But how much is enough? In reality, we don’t need as much Sprinkle with 2 tablespoons chia protein as we think we do – the chart below shows the Recommended seeds (3.5g) or 2 tablespoons Dietary Allowance based on age and sex. ground flaxseeds (3g) Recommended Dietary Allowance for Protein 1 cup soy milk (8g) › Grams of Protein Needed Daily Total: 17-17.5g protein Children ages 1–3 13 LUNCH Children ages 4–8 19 2 slices whole wheat bread (7.2g) Children ages 9–13 34 1 cup raw spinach leaves (1g) Girls ages 14–18 46 tossed with ½ ounce walnuts (2g), ½ cup mushrooms (1g). Boys ages 14–18 52 Dress lightly with olive oil and Women ages 19–70+ 46 balsamic vinegar. Men ages 19–70+ 56 › Total: 11.2g protein (You can also multiply your weight in pounds by .36 to get your daily protein need in grams. For instance, if you weigh DINNER 150 pounds, aim for about 54g of protein.) 2 oz. whole grain pasta (7g) Sauté ½ cup tomatoes (.75g), toss Getting enough protein on a plant-based diet can be easy and delicious. with ½ cup chickpeas (8g), Choose from a bounty of nuts and seeds, legumes such as beans and 2 tablespoons olive oil, fresh basil lentils, soy foods, brown rice and other whole grains, and of course leaves, salt and pepper. -

The Protein Debate – Understanding the Movement to Plant-Based Eating
The Protein Debate – understanding the movement to plant-based eating Kellogg Rural Leadership Programme Course 41 2020 Kate Downie-Melrose 1 I wish to thank the Kellogg Programme Investing Partners for their continued support: Disclaimer In submitting this report, the Kellogg Scholar has agreed to the publication of this material in its submitted form. This report is a product of the learning journey taken by participants during the Kellogg Rural Leadership Programme, with the purpose of incorporating and developing tools and skills around research, critical analysis, network generation, synthesis and applying recommendations to a topic of their choice. The report also provides the background for a presentation made to colleagues and industry on the topic in the final phase of the Programme. Scholars are encouraged to present their report findings in a style and structure that ensures accessibility and uptake by their target audience. It is not intended as a formal academic report as only some scholars have had the required background and learning to meet this standard. This publication has been produced by the scholar in good faith on the basis of information available at the date of publication, without any independent verification. On occasions, data, information, and sources may be hidden or protected to ensure confidentially and that individuals and organisations cannot be identified. Readers are responsible for assessing the relevance and accuracy of the content of this publication & the Programme or the scholar cannot be liable for any costs incurred or arising by reason of any person using or relying solely on the information in this publication. -

A STUDY of PROTEIN EXTRACT from SOYBEANS with REFERENCE to ITS USE in FOOD ' the Soybean (Soja Max (L.) Piper) Is Unique Among P
A STUDY OF PROTEIN EXTRACT FROM SOYBEANS WITH REFERENCE TO ITS USE IN FOOD ' By SYBIL WOODRUFF, chief in foods, EVELYN CHAMBERS, graduate student, and HELEN KLAAS, first assistant in home economics, Illinois Agricultural Experiment Station INTRODUCTION The soybean (Soja max (L.) Piper) is unique among plant foods in that it contains about 40 percent of protein. The supply of soybeans gives promise of being abundant, too; Illinois produced in 1937 a little less than 22 million bushels, an increase of about 27 percent over her crop for 1936. Here is a potentially important source of protein, little of which is now being consumed as food. Some people are ad- vocating that the United States extend the food uses of soybeans for the sake of obtaining inexpensive protein. This might be done in either of two ways; by utilizing the beans themselves or by making from the beans a protein concentrate which could take its place with other protein-rich foods. The purpose of this study was to find a simple method of extracting semipure protein from soybeans and then to determine whether the protein substance could be used advantageously in the preparation of food. REVIEW OF LITERATURE The literature relating to soybean proteins and their physical be- havior is not extensive and is somewhat confused by the variety of laboratory methods which have been used in extracting the proteins. Moreover, papers bearing on the topic have not been concerned with the uses of the protein in food technology but rather with its chemical nature. There is little in the literature concerning the methods that are being used for removing crude soybean protein for the manufacture of plastics. -

Meat Proteins in Human Nutrition
MEAT P80T€IN'S IN HUMAN NUTRITION J. KASTELIC Before I can begin to discuss the many questions which are implied in the title of this discussion I must beg your forbearance. I must clearly specify that I am appearing before you as a student of protein nutrition and not as an authority. This is not meant to be an apology. It is a statement which is not only pmdeut but entire1.y appropriate. And I must clear a bit of underb-msli amy before I give the su'oject before us specific consideration, ;^or I can be certain none of you came here only to listen to me reiterate the resalts of nitrogen balance studies published in the literature or to look at relmoductions of tables of data and of graphs which describe the results of the many biological value determinations of proteins that have been obt,ained frola laboratory animal experimentation with which so many of you are now familiar. It would be equally superfluous to call attention to the tremendous volume of literature that is now being published about the underlying eco- nomic, social, technological and medical problems which are confronting man in so many parts of the world today where food production is only sufficient to mainkin life. !here we were once so provincially concerned with vitamin defi- ciency diseases, we now appear to be rather singularly concerned about pro- tein malnutrition and how m might best cope with protein malnutrition in the human obliged to subsist on diets composed of protein-deficient foods. If there is still some controversy about the relative merits of proteins from plant and animal sources in 'numan nutrition it must stem from vexing economic considerations, meat animal production capabilities and problems associated with processing and storage; not fmm a lack of an appreciation of the nutritional role and functions of the amino acids which are essential to life.