Musculoskeletal System Procedures Performed More Than 12000 Times
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post–Vertebral Augmentation Back Pain: ORIGINAL RESEARCH Evaluation and Management
Post–Vertebral Augmentation Back Pain: ORIGINAL RESEARCH Evaluation and Management S. Kamalian BACKGROUND AND PURPOSE: Vertebral augmentation is an established treatment for painful osteo- R. Bordia porotic vertebral fractures of the spine. Nevertheless, patients may continue to have significant back pain afterward. The purpose of this study was to assess the source of persistent or recurrent back pain A.O. Ortiz following vertebral augmentation. MATERIALS AND METHODS: Our institutional review board approved this study. We evaluated 124 consecutive patients who underwent vertebral augmentation for painful osteoporotic vertebral frac- tures. All patients were evaluated after 3 weeks, 3 months, and 1 year following their procedure. Patients with any type of back pain after their procedure were examined under fluoroscopy. RESULTS: Thirty-four of 124 (27%) patients were men, and 90/124 (73%) were women. Persistent or recurrent back pain, not due to a new fracture or a failed procedure, was present in 29/124 (23%) patients. The source of pain was most often attributed to the sacroiliac and/or lumbar facet joints (25/29 or 86%). Seventeen of 29 (59%) patients experienced immediate relief after facet joint injection of a mixture of steroid and local anesthetic agents. The remaining 12 (41%) had relief after additional injections. Ten (34%) patients ultimately required radio-frequency neurolysis for long-term relief. CONCLUSIONS: Back pain after vertebral augmentation may not be due to a failed procedure but rather to an old or a new pain generator, such as an irritated sacroiliac or lumbar facet joint. This is of importance not only for further pain management of these patients but also for designing trials to compare the efficacy of vertebral augmentation to other treatments. -

Unicompartmental Knee Replacement
This is a repository copy of Unicompartmental Knee Replacement. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/120113/ Version: Accepted Version Article: Takahashi, T, Pandit, HG orcid.org/0000-0001-7392-8561 and Phil, D (2017) Unicompartmental Knee Replacement. Journal of Arthroscopy and Joint Surgery, 4 (2). pp. 55-60. ISSN 0021-8790 https://doi.org/10.1016/j.jajs.2017.08.009 © 2017 International Society for Knowledge for Surgeons on Arthroscopy and Arthroplasty. Published by Elsevier, a division of RELX India, Pvt. Ltd. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Accepted Manuscript Title: Unicompartmental Knee Replacement Author: Tsuneari Takahashi PII: S2214-9635(17)30041-X DOI: http://dx.doi.org/doi:10.1016/j.jajs.2017.08.009 Reference: JAJS 97 To appear in: Authors: Hemant G. -

Procedure Codes
NEW YORK STATE MEDICAID PROGRAM ORDERED AMBULATORY PROCEDURE CODES Ordered Ambulatory Procedure Codes Table of Contents GENERAL INFORMATION ---------------------------------------------------------------------------------------------------------------2 LABORATORY SERVICES INFORMATION-----------------------------------------------------------------------------------------2 RADIOLOGY INFORMATION------------------------------------------------------------------------------------------------------------3 MMIS MODIFIERS --------------------------------------------------------------------------------------------------------------------------6 RADIOLOGY SERVICES------------------------------------------------------------------------------------------------------------------7 DIAGNOSTIC RADIOLOGY (DIAGNOSTIC IMAGING) ------------------------------------------------------------------------7 DIAGNOSTIC ULTRASOUND SERVICES- -------------------------------------------------------------------------------------- 20 RADIOLOGIC GUIDANCE....................................................................................................................................25 BREAST, MAMMOGRAPHY --------------------------------------------------------------------------------------------------------- 26 BONE/JOINT STUDIES --------------------------------------------------------------------------------------------------------------- 26 RADIATION ONCOLOGY SERVICES --------------------------------------------------------------------------------------------- 27 NUCLEAR -

WARD-DOCUMENT-2020.Pdf (1.324Mb)
3D Printing Impact on the Orthopedic Shoulder Replacement Global Supply Chain The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Ward, Abner. 2020. 3D Printing Impact on the Orthopedic Shoulder Replacement Global Supply Chain. Master's thesis, Harvard Extension School. Citable link https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37365004 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA 3D Printing Impact on the Orthopedic Shoulder Replacement Global Supply Chain Abner M. Ward, MD, MBA, FACS, FAAOS A Thesis in the Field of Biotechnology Management for the Degree of Master of Liberal Arts in Extension Studies Harvard University May 2020 Copyright 2020 [Abner Ward] Abstract The goal of this work was to investigate a novel new technology being used to improve total shoulder replacements in patients with difficult to treat anatomy. The new technology is the use of three-dimensional (3D) implant creations that can be tailored to a patient’s specific shoulder defects as opposed to shelf, standard size implants. The project will help provide management direction to improve the efficiency in the global supply system so that surgeons in various parts of the world may have access to surgical components in the shortest time without significant delay. The study findings were that hindrances to 3D adoption for just-in-time surgical usage primarily include difficulties with sterilization and lack of a global validation metric when performed at multiple international centers, as opposed to one location in a single country. -

Vertebral Augmentation ICD 9 Codes: Osteoporosis 733. 0, Vertebra
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Vertebral Augmentation ICD 9 Codes: Osteoporosis 733. 0, Vertebral Fracture closed 805.8, Pathological fracture of Vertebrae 733.13 Vertebral augmentation, known as vertebroplasty and kyphoplasty, is a minimally invasive procedure that is used to treat vertebral fractures. Vertebral fractures are the most common skeletal injury associated with osteoporosis, and it is estimated that more than 750,000 occur annually in the United States.1 Up to one quarter of people over 50 years of age will have at least one vertebral fracture in their life time secondary to osteoporosis.2 According to the World Health Organization (WHO), the operational definition of osteoporosis is a bone density measure >2.5 standard deviations (SD) below the mean of young healthy adults of similar race and gender.3 Primary osteoporosis is related to the changes in postmenopausal women secondary to reduction of estrogen levels and related to age-related loss of bone mass. Secondary osteoporosis is the loss of bone caused by an agent or disease process. 1,4 (See Osteoporosis SOC) The severity of vertebral fractures can be assessed by the Genat semiquantitative method. Commonly used by radiologists, this scale assesses the severity of the fracture visually and has been shown to be reliable.5 Genat Semiquantitive Grading System for Vertebral Deformity5 Grade 0- normal vertebral height Grade 1- minimal fracture- 20-25% height decrease Grade 2- moderate fracture- 25-40% height decrease Grade 3-severe- >40% height decrease Standard methods of diagnosing vertebral fractures are imaging, including the following: CT scan, MRI, and radiography. -

Evicore Musculoskeletal Imaging Guidelines
CLINICAL GUIDELINES Musculoskeletal Imaging Policy Version 2.1 Effective October 1, 2020 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2020 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2020 eviCore healthcare. All rights reserved. Musculoskeletal Imaging Guidelines V2.1 Musculoskeletal Imaging Guidelines Procedure Codes associated with Musculoskeletal Imaging 3 MS-1: General Guidelines 5 MS-2: Imaging Techniques 8 MS-3: 3D Rendering 12 MS-4: Avascular Necrosis (AVN)/Osteonecrosis 13 MS-5: Fractures 16 MS-6: Foreign Body 20 MS-7: Ganglion Cysts 22 MS-8: Gout/Calcium Pyrophosphate Deposition Disease (CPPD)/ Pseudogout/Chondrocalcinosis 24 MS-9: Infection/Osteomyelitis 26 MS-10: Soft Tissue Mass or Lesion of Bone 29 MS-11: Muscle/Tendon Unit -
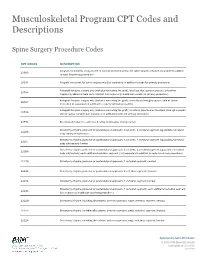
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Effectiveness of Cementoplasty for Vertebral Augmentation in Multiple Myeloma: a Case Series
WCRJ 2017; 4 (2): e882 EFFECTIVENESS OF CEMENTOPLASTY FOR VERTEBRAL AUGMENTATION IN MULTIPLE MYELOMA: A CASE SERIES G. TESTA1, M. PRIVITERA1, T. FIDILIO1, G. DI STEFANO1, A. VESCIO1, G. D’ANGELO2, V. PAVONE1 1Department of Orthopedics and Traumatologic Surgery, AOU Policlinico-Vittorio Emanuele, University of Catania, Catania, Italy 2Department of Human Pathology in Adult and Developmental Age “Gaetano Barresi” – Unit of Paediatrics, University of Messina, Messina, Italy Abstract – Objective: Multiple myeloma (MM) is a neoplasm characterized by the proliferation of somatically mutated plasma cells that tend to expand within the bone marrow and affect mul- tiple locations throughout the bone marrow. When it is located in vertebral areas it causes bone lesions with pain, kyphosis, walking impairments, and disability. Different types of treatments are available. The goal of this study is to report our experience regarding the treatment of vertebral fractures from multiple myeloma using cementoplasty. Patients and Methods: From January 2012 to December 2015, 38 patients with multiple mye- loma and multilevel vertebral fractures were treated. Seventeen patients underwent conservative treatment (group 1), and 21 patients underwent vertebral augmentation with percutaneous ce- mentoplasty (group 2). Both groups were clinically evaluated at 1, 6 and 12 months using a visual analogic scale (VAS) for pain, SF-36 and ODI Score Questionnaires. Radiographic evaluation was performed to verify the quality of cementoplasty and complications. Results: Mean follow-up was 23.7 months. Mean VAS score in group 1 decreased from 7.1 pre-operatively to 3.9 at final follow-up (p<0.05). In group 2, this score decreased from 7.3 pre-op- eratively to 2.3 at final follow-up (p<0.05). -

Procedure Coding in ICD-9-CM and ICD- 10-PCS
Procedure Coding in ICD-9-CM and ICD- 10-PCS ICD-9-CM Volume 3 Procedures are classified in volume 3 of ICD-9-CM, and this section includes both an Alphabetic Index and a Tabular List. This volume follows the same format, organization and conventions as the classification of diseases in volumes 1 and 2. ICD-10-PCS ICD-10-PCS will replace volume 3 of ICD-9-CM. Unlike ICD-10-CM for diagnoses, which is similar in structure and format as the ICD-9-CM volumes 1 and 2, ICD-10-PCS is a completely different system. ICD-10-PCS has a multiaxial seven-character alphanumeric code structure providing unique codes for procedures. The table below gives a brief side-by-side comparison of ICD-9-CM and ICD-10-PCS. ICD-9-CM Volume3 ICD-10-PCS Follows ICD structure (designed for diagnosis Designed and developed to meet healthcare coding) needs for a procedure code system Codes available as a fixed or finite set in list form Codes constructed from flexible code components (values) using tables Codes are numeric Codes are alphanumeric Codes are 3-4 digits long All codes are seven characters long ICD-9-CM and ICD-10-PCS are used to code only hospital inpatient procedures. Hospital outpatient departments, other ambulatory facilities, and physician practices are required to use CPT and HCPCS to report procedures. ICD-9-CM Conventions in Volume 3 Code Also In volume 3, the phrase “code also” is a reminder to code additional procedures only when they have actually been performed. -

Clinical Guidelines
CLINICAL GUIDELINES Joint Services Guidelines Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Large Joint Services CMM-311: Knee Replacement/Arthroplasty 3 CMM-312: Knee Surgery-Arthroscopic and Open Procedures 14 CMM-313: Hip Replacement/Arthroplasty 35 CMM-314: Hip Surgery-Arthroscopic and Open Procedures 46 CMM-315: Shoulder Surgery-Arthroscopic and Open Procedures 47 CMM-318: Shoulder Arthroplasty/ Replacement/ Resurfacing/ Revision/ Arthrodesis 62 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 69 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-311: Knee Replacement/Arthroplasty CMM-311.1: Definition 4 CMM-311.2: General Guidelines 5 CMM-311.3: Indications and Non-Indications 5 CMM-311.4 Experimental, Investigational, or Unproven 9 CMM-311.5: Procedure (CPT®) Codes 10 CMM-311.6: References 10 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 3 of 69 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-311.1: Definition -

Predictors of Outcome Following Standardized Rehabilitation for Patients with Shoulder Pain
University of Kentucky UKnowledge Theses and Dissertations--Rehabilitation Sciences Rehabilitation Sciences 2013 Predictors of Outcome Following Standardized Rehabilitation for Patients with Shoulder Pain Stephanie D. Moore University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Moore, Stephanie D., "Predictors of Outcome Following Standardized Rehabilitation for Patients with Shoulder Pain" (2013). Theses and Dissertations--Rehabilitation Sciences. 15. https://uknowledge.uky.edu/rehabsci_etds/15 This Doctoral Dissertation is brought to you for free and open access by the Rehabilitation Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Rehabilitation Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained and attached hereto needed written permission statements(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine). I hereby grant to The University of Kentucky and its agents the non-exclusive license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. I agree that the document mentioned above may be made available immediately for worldwide access unless a preapproved embargo applies. -

Vertebroplasty and Percutaneous Vertebral Augmentation
Medicare Part C Medical Coverage Policy Vertebroplasty and Percutaneous Vertebral Augmentation Origination Date: December 16, 2002 Vertebroplasty August 20, 2003 Kyphoplasty Review Date: June 17, 2020 Next Review: June, 2022 ***This policy applies to all Blue Medicare HMO, Blue Medicare PPO, Blue Medicare Rx members, and members of any third-party Medicare plans supported by Blue Cross NC through administrative or operational services. *** DESCRIPTION OF PROCEDURE OR SERVICE Vertebroplasty Percutaneous vertebroplasty is a therapeutic, interventional radiologic procedure, which consists of the injection of a biomaterial (usually polymethylmethacrylate- bone cement) under imaging guidance (either fluoroscopy or CT) into a cervical, thoracic or lumbar vertebral body lesion for the relief of pain and the strengthening of bone. Percutaneous Vertebral Augmentation This is also known as balloon-assisted Percutaneous Vertebroplasty or Kyphoplasty. The procedure is similar to percutaneous vertebroplasty in that stabilization of the collapsed vertebra is accomplished by the injection of the same biomaterial into the body of the vertebra. The primary difference is that the fracture is partially reduced with the insertion of an inflatable balloon tamp. Once inflated, the balloon tamp (plug) restores some height to the vertebral body, while creating a cavity that is filled with bone cement. POLICY STATEMENT Coverage will be provided for vertebroplasty or percutaneous vertebral augmentation when it is determined to be medically necessary because the