Measuring the Inter and Intraspecific Sexual Shape Dimorphism And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Barcoding and Morphology Reveal Three Cryptic Species of Anania
Systematic Entomology (2012), 37, 686–705 DNA barcoding and morphology reveal three cryptic species of Anania (Lepidoptera: Crambidae: Pyraustinae) in North America, all distinct from their European counterpart ZHAOFU YANG1,9, JEAN-FRANC¸ OIS LANDRY2,LOUIS HANDFIELD3, YALIN ZHANG1,M.ALMASOLIS4, DANIEL HANDFIELD5, BRIAN G. SCHOLTENS6, MARKO MUTANEN7, MATTHIAS NUSS8 and PAUL D. N. HEBERT9 1Key laboratory of Plant Protection Resources and Pest Management, Ministry of Education; Entomological Museum, Northwest A&F University, Yangling, China, 2Agriculture and Agri-Food Canada, Eastern Cereal and Oilseed Research Centre, C.E.F., Ottawa, Ontario K1A 0C6, Canada, 3133 rue Messier, #301, Mont-Saint-Hilaire, Quebec´ J3H 2W8, Canada, 4Systematic Entomology Laboratory, USDA, c/o Smithsonian Institution, National Museum Natural History, Washington, DC 20013-7012, U.S.A., 5Chemin des Grands Coteaux, Saint-Mathieu-de-Beloeil, Quebec,´ Canada, 6Department of Biology, College of Charleston, SC, U.S.A., 7Department of Biology, University of Oulu, Zoological Museum, Oulu, Finland, 8Museum of Zoology, Senckenberg Natural History Collections Dresden, Konigsbr¨ ucker¨ Landstrasse 159, 01109 Dresden, Germany and 9Biodiversity Institute of Ontario, University of Guelph, Guelph, Ontario N1G 2W1, Canada Abstract. Anania coronata (Hufnagel), a Holarctic species of pyraustine crambid moth, has long been treated as having two geographically separated subspecies – the nominotypical Anania coronata in the Palaearctic Region and Anania coronata tertialis (Guenee)´ in the Nearctic Region. Maximum likelihood and Bayesian inference analysis of mitochondrial DNA barcodes both recover four well-supported, reciprocally monophyletic groups within Anania coronata. Qualitative and quantitative analyses of genital structures reveal diagnostic differences that correspond to the four barcode lineages. On the basis of both molecular and morphological evidence, we conclude that Anania coronata is actually a complex of four species. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -

Diagnoses and Remarks on the Genera of Tortricidae (Lepidoptera)
Acta zoologica cracoviensia, 58(2): 195-252, Kraków, 31 December, 2015 Ó Institute of Systematics and Evolution of Animals, Pol. Acad. Sci., Kraków doi:10.3409/azc.58_2.195 DiagnosesandremarksonthegeneraofTortricidae (Lepidoptera). Part3.Archipini JózefRAZOWSKI Received:15July2015.Accepted:21December2015.Availableonline:31December2015. RAZOWSKI J. 2015. Diagnoses and remarks on the genera of Tortricidae (Lepidoptera). Part3.Archipini. Actazool.cracov., 58(2): 195-252. Abstract. Comparative diagnoses, redescriptions, and remarks are presented on the genera of the tribe Archipini. Original references, type species, synonyms, numbers of known species, and zoogeographic regions are provided. Merophyas COMMON, 1964, is synoni- mizedwith Clepsis GUENÉE,1845. Keywords:Lepidoptera,Tortricidae,Archipini,genera,comparativediagnoses. Józef RAZOWSKI, Insitute of the Systematics and Evolution of Animals, Polish Academy * ofSciences, S³awkowska17, 31-016Kraków,Poland. E-mail:[email protected] I.INTRODUCTION The number of genera of Tortricidae has increased dramatically over last 50 years; by 2007 there were over 1630 described genera, including synonyms. Many of the older de- scriptions are scattered throughout the literature, and because there are few larger synthetic treatments of the tortricids for most major biogeographic regions, this large number of taxa complicates considerably the work of taxonomists on the faunas of poorly known regions of the planet. In addition, characters that define many of the genera are not clearly articu- lated. The distribution of many genera is still insufficiently known, and this shortcoming frequently results in unexpected findings, e.g., the discovery of Afrotropical genera in the Neotropics. These types of discoveries may cause confusion for specialists that focus on thefaunaofasinglegeographicregion. The literature abounds with re-descriptions and diagnoses of tortricid genera, but many are rather short, frequently lacking comparisons with similar or related taxa. -

To Download the Food Plant Database in PDF Format
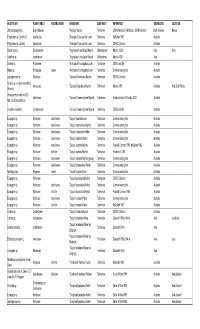
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Abelia spathulata Siebold & Zucc. Caprifoliaceae Acleris askoldana (Christoph) Tortricinae Yasuda 1972 Asia Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982; Nasu & Yasuda 1993 Asia Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota nigrocervina Walsingham Tortricinae MacKay 1962a North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota rostrana (Walker) Tortricinae Heinrich 1921 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Archips micaceana (Walker) Tortricinae Pholboon 1965; Kuroko & Lewvanich 1993 Asia Moench Abelmoschus esculentus (L.) Malvaceae Archips philippa (Meyrick) Tortricinae BMNH collection Asia India Moench Abelmoschus esculentus (L.) Malvaceae Homona tabescens (Meyrick) Tortricinae Yunus & Ho 1980 Asia Malaysia Moench Abelmoschus esculentus Moench Malvaceae Crocidosema plebejana Zeller Olethreutinae Fletcher 1932 Asia India (as Hibiscus) Abelmoschus esculentus Moench Thaumatotibia leucotreta (Meyrick) (as Malvaceae Olethreutinae Whittle 1984 Africa (as Hibiscus) Cryptophlebia) Abies alba Mill. Pinaceae Acleris variana (Fernald) Tortricinae Meyrick MS 1938 North America Abies alba Mill. Pinaceae Archips oporana (Linnaeus) Tortricinae Bradley et al. 1973 Europe Abies -

Independent Life History Evolution Between Generations of Bivoltine Species: a Case Study of Cyclical Parthenogenesis
Oecologia DOI 10.1007/s00442-017-3824-5 POPULATION ECOLOGY – ORIGINAL RESEARCH Independent life history evolution between generations of bivoltine species: a case study of cyclical parthenogenesis Glen R. Hood1 · James R. Ott2 Received: 6 September 2016 / Accepted: 19 January 2017 © Springer-Verlag Berlin Heidelberg 2017 Abstract Successive generations of bi- and multivoltine relationships, interpreted in the context of the model frame- species encounter differing biotic and abiotic environments work, suggest that within each generation selection has intra-annually. The question of whether selection can inde- independently molded the relationships relating body size pendently adjust the relationship between body size and to potential fecundity and potential reproductive effort in components of reproductive effort within successive gen- B. treatae. The conceptual framework is broadly applicable erations in response to generation-specific environmental to comparisons involving the alternating generations of bi- variation is applicable to a diversity of taxa. Herein, we and multivoltine species. develop a conceptual framework that illustrates increas- ingly independent life history adjustments between suc- Keywords Body size · Complex life cycles · Cynipidae · cessive generations of taxa exhibiting complex life cycles. Fecundity · Heterogony We apply this framework to the reproductive biology of the gall-forming insect, Belonocnema treatae (Hymenop- tera: Cynipidae). This bivoltine species expresses cyclical Introduction parthenogenesis in -

A-Razowski.Vp:Corelventura
Acta zoologica cracoviensia, 59(2) 2016 ISSN 2299-6060, e-ISSN 2300-0163 Kraków, 30 December, 2016 doi:10.3409/azc.59_2.89 Ó Institute of Systematics and Evolution of Animals, PAS Diagnoses and remarks on the genera of Tortricidae (Lepidoptera). Part 4. Cnephasiini, Ceracini, Atteriini, Sparganothini and Euliini Józef RAZOWSKI Received: 04 October 2016. Accepted: 22 November 2016. Available online: 02 December 2016. RAZOWSKI J. 2016. Diagnoses and remarks on the genera of Tortricidae (Lepidoptera). Part 4. Cnephasiini, Ceracini, Atteriini, Sparganothini and Euliini. Acta zool. cracov.,59(2): 89-151. Abstract. Diagnoses, redescriptions, and remarks are presented on the genera that com- prised the five tortricid tribes Cnephasiini (19 genera), Ceracini (4 genera), Atteriini (9 genera), Sparganothini (20 genera), and Euliini (173 genera). Original references, type species, type localities, synonyms, and zoogeographic regions are provided. Moronata is a synonym of Pseudapina. Raisapoana, omitted in Archipini is added in the appendix. Key words: Lepidoptera, Tortricidae, genera, diagnoses, descriptions * Józef RAZOWSKI, Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, S³awkowska 17, 31-016 Kraków, Poland. E-mail: [email protected] I. INTRODUCTION The number of genera of Tortricidae has increased dramatically over last 40 years; by 2007 there were over 1630 described genera, including synonyms. Many of the older de- scriptions are scattered throughout the literature, and because there are few larger synthetic treatments of the tortricids for most major biogeographic regions, this large number of taxa complicates considerably the work of taxonomists on the faunas of poorly known regions of the planet. In addition, characters that define many of the genera are not clearly articu- lated. -
Sorted by Moth Species
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Jatropha gossypifolia Euphorbiaceae "Amorbia" depicta Tortricinae CSIRO Mexican Field Station; USNM collection North America Meixco Polystichum sp. ("prolifera") Aspidiaceae "Anisogona" placoxantha Lower Tortricinae McQuillan 1992 Australia Polystichum sp. (as fern) Aspidiaceae "Anisogona" placoxantha Lower Tortricinae CSIRO Collection Australia Cordia myxa L. Boraginaceae "Argyroploce" cenchropis Meyrick Olethreutinae Meyrick 1920a Asia India Acanthus sp. Acanthaceae "Argyroploce" vinculigera Meyrick Olethreutinae Meyrick 1939 Asia Banksia sp. Proteaceae "Arotrophora" cosmoplaca Lower Tortricinae CSIRO card file Australia Hakea sp. Proteaceae leaves "Arotrophora" cosmoplaca Lower Tortricinae Common rearing files Australia Leptospermum sp. Myrtaceae "Cacoecia" desmotana Meyrick Tortricinae CSIRO Collection Australia Senecio sp. (as plant resembling Asteraceae "Cacoecia" jugicolana Meyrick Tortricinae Meyrick 1881 Australia New South Wales Senecio) Osteospermum ecklonis (DC.) Asteraceae "Cacoecia" mnemosynana Meyrick Tortricinae Herbison-Evans & Crossley 2003 Australia Norl. (as Dimorphotheca) Goodenia ovata Sm. Goodeniaceae "Cacoecia" mnemosynana Meyrick Tortricinae CSIRO card file Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" ceramica Lower Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" cnaphalodes Meyrick Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" constrictana -

Sexual Dimorphism and Population Differentiation in the Chilean
Revista Brasileira de Entomologia 61 (2017) 365–369 REVISTA BRASILEIRA DE Entomologia A Journal on Insect Diversity and Evolution www.rbentomologia.com Systematics, Morphology and Biogeography Sexual dimorphism and population differentiation in the Chilean Neotropical moth Macaria mirthae (Lepidoptera, Geometridae): a wing geometric morphometric example a,b,∗ a Hugo A. Benítez , Héctor A. Vargas a Universidad de Tarapacá, Facultad de Ciencias Agronómicas, Departamento de Recursos Ambientales, Arica, Chile b Cambridge University, Museum of Zoology, Cambridge, United Kingdom a b s t r a c t a r t i c l e i n f o Article history: Sexual shape dimorphism is the differentiation of male and female organisms based on their shape Received 16 April 2017 variation; this definition was proposed for the use of geometric morphometrics analysis where the Accepted 20 June 2017 geometric features of the shape are analyzed without the influence of the size. Macaria mirthae Available online 5 July 2017 (Lepidoptera: Geometridae) is a moth that inhabits different valleys in the north of Chile principally Associate Editor: Livia Pinheiro associated to Acacia macracantha and lately Leucaena leucocephala both trees of the Fabaceae family. The Sexual dimorphism was analyzed in this species in order to corroborate studies on the use of wing Keywords: as a sexual differentiation trait, and specific influence of localities was also evaluated. A clear shape Wing shape variation was found where the male wings are more contracted compared to female wings. A climate Sexual dimorphism Geometridae influence is also suggested that could differentiate the wing shape from the individuals that inhabit two different valleys in the neotropical region of the north of Chile. -

Redalyc.New and Interesting Portuguese Lepidoptera Records from 2014 (Insecta: Lepidoptera)
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Corley, M. F. V.; Rosete, J.; Romão, F.; Dale, M. J.; Marabuto, E.; Maravalhas, E.; Pires, P. New and interesting Portuguese Lepidoptera records from 2014 (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 43, núm. 172, diciembre, 2015, pp. 583-613 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45543699006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Revta. lepid., 43 (172), diciembre 2015: 583-613 eISSN: 2340-4078 ISSN: 0300-5267 New and interesting Portuguese Lepidoptera records from 2014 (Insecta: Lepidoptera) M. F. V. Corley, J. Rosete, F. Romão, M. J. Dale, E. Marabuto, E. Maravalhas & P. Pires Abstract 38 species are added to the Portuguese Lepidoptera fauna and three species deleted, mainly as a result of fieldwork undertaken by the authors and others in 2014. In addition, second and third records for the country and new food-plant data for a number of species are included. A summary of recent papers affecting the Portuguese fauna is included. KEY WORDS: Insecta, Lepidoptera, distribution, Portugal. Novos e interesantes registos portugueses de Lepidoptera de 2014 (Insecta: Lepidoptera) Resumo Como resultado do trabalho de campo desenvolvido pelos autores principalmente no ano de 2014, são adicionadas 38 espécies de Lepidoptera para a fauna de Portugal e tres são retiradas da lista nacional. -

Revision of the Genus Ditula (Lepidoptera: Tortricidae) with Description of a New Species
Revision of the genus Ditula (Lepidoptera: Tortricidae) with description of a new species Knud Larsen Abstract. In this paper the genus Ditula Stephens, 1829 is revised. The new species Ditula bartoniana n. sp. from south west Cyprus is described and the taxon Ditula saturana (Turati, 1913) is sunk as a synonym of the common species Ditula angustiorana (Haworth, 1828). The species Ditula joannisiana (Ragonot, 1888) is discussed but for the moment kept in the genus Ditula. The distribution of the genus and the species in the genus is exposed and discussed. Samenvatting. Revisie van het genus Ditula (Lepidoptera: Tortricidae) met beschrijving van een nieuwe soort Het genus Ditula Stephens, 1829 wordt bewerkt. De nieuwe soort Ditula bartoniana sp. n. uit Zuidwest-Cyprus wordt beschreven en het taxon Ditula saturana (Turati, 1913) wordt gesynonymiseerd met de gewone soort Ditula angustiorana (Haworth, 1828). De soort Ditula joannisiana (Ragonot, 1888) wordt besproken en voorlopig in het genus Ditula behouden. De verspreiding van de het genus Ditula en de soorten erin wordt besproken. Résumé. Révision du genre Ditula (Lepidoptera: Tortricidae) et description d’une espèce nouvelle Le genre Ditula Stephens, 1829 est révisé et une espèce nouvelle, Ditula bartoniana n. sp., est décrite du sud-ouest de l’île de Chypre. Le taxon Ditula saturana (Turati, 1913) est mis en synonymie avec l’espèce commune Ditula angustiorana (Haworth, 1828). L’espèce Ditula joannisiana (Ragonot, 1888) est discutée et maintenue dans le genre Ditula provisoirement. La répartition du genre Ditula et de ses espèces est discutée. Key words. Ditula bartoniana – Descriptions – Systematics – Faunistics – Cyprus. Larsen K.: Røntoftevej 33, DK-2870 Dyssegaard, Denmark. -

Lepidópteros De Los Espacios Naturales Protegidos Del Litoral De Huelva (Macro Y Microlepidoptera) Manuel Huertas Dionisio
Lepidópteros de los Espacios Naturales Protegidos del Litoral de Huelva (Macro y Microlepidoptera) Manuel Huertas Dionisio Córdoba, Marzo de 2007 ISSN 1699-2679 Monográfico nº 2 Sociedad Andaluza de Entomología Publicación periódica de la Sociedad Andaluza de Entomología Apartado de correos 3.086. 14080 CÓRDOBA. Telf. 957293086 / 676343151 [email protected] DL. CO-442-01 - RACDP Nº 242 - RNA Nº 145.295 - RAA Nº 24 – RMA Nº 1235/2005 MONOGRÁFICO Nº 2 marzo 2007 ISSN 1699-2679 La Sociedad Andaluza de Entomología -SAE- nace en 2001 por transformación de la Sociedad Entomológica Cordobesa - SOCECO- fundada en 1995 de acuerdo con la Ley de Asociaciones de 24 de diciembre de 1964 y sin ánimo de lucro, cuyo objetivo es la conservación y estudio de los insectos y difusión de la ciencia de la Entomología a través de sus publicaciones. PRESIDENTE José Machado Aragonés SECRETARIO Francisco Manuel Cobos García EDITOR SAE COMITÉ DE REDACCIÓN José Machado Aragonés ● Francisco Manuel Cobos García Joaquín Fernández de Córdova Villegas ● Alfonso Roldán Losada Juan Manuel Fernández Maestre ● Fernando J. Fuentes García Juan Francisco López Caro ● Antonio Luna Murillo Antonio Verdugo Páez ● Manuel Huertas Dionisio MAQUETACIÓN Y DISEÑO Antonio Luna Murillo PORTADA Malacosoma laurae Lajonquière, 1977 Fotografía: Rafael GARCÍA RODRÍGUEZ COLABORAN Esta publicación es recogida en las siguientes bases de datos: INIST, Zoological Record, DIALNET. Los autores se responsabilizan de las opiniones e información contenida en los artículos y comunicaciones. Se autoriza la reproducción total o parcial de este Boletín por cualquier persona o entidad con el único fin de la difusión cultural o científica, sin fines lucrativos y citando la fuente.