Adenylate-Forming En
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curriculum Vitae Personal
CURRICULUM VITAE PERSONAL: Name: Elizabeth J. Goldsmith Address: Dept. of Biochemistry UT Southwestern Medical Center 5323 Harry Hines Blvd. Dallas, Texas 75390-8816 Telephone: (214) 645-6376 FAX: (214) 645-6387 E-mail: [email protected] EDUCATION: 1967 B. S., Chemistry University of California, Los Angeles 1971 Ph.D., Physical Chemistry University of California, Los Angeles ACADEMIC POSITIONS: 1998 - Professor, Biochemistry, The University of Texas Southwestern Medical Center at Dallas, Texas 1993 - 1998 Associate Professor, Biochemistry, The University of Texas Southwestern Medical Center at Dallas, Texas 1986 - 1993 Assistant Professor, Biochemistry, The University of Texas Southwestern Medical Center at Dallas, Texas 1980 - 1986 Assistant Research Professor, University of California, San Francisco, CA Mechanism of activation of phosphorylase by saccharides Structure of Oligosaccharides Carboxypeptidase mutants 1976-1980 Assistant Research Professor, University of California, Los Angeles Crystallographic studies of glutamine synthetase 1974-1975 Acting Assistant Professor, Postdoctoral Fellow University of California, Los Angeles Crystallographic studies of aldolase and RNA polymerase Crystallization and preparation of heavy atom derivatives Laboratory of David Eisenberg 1 1972-1973 Postdoctoral fellow, MRC Laboratory for Molecular Biology Cambridge, England Structure of carbonmonoxyhemoglobin at 2 Å Laboratory of Max Perutz AWARDS AND FELLOWSHIPS: 1963 Dean's List, UCLA 1967 Honors in Chemistry, UCLA 1971 Graduate Woman of the Year, UCLA 1972 American Assoc. of University Women Fellowship 1973 NIH Postdoctoral Fellowship 1974 Celeste Durand Rogers Fellowship 1975 NIH Career Development Award PROFESSIONAL SOCIETIES: American Crystallographic Association Protein Society Biophysical Society American Society of Biological Chemists RESEARCH INTERESTS: Conformational regulation of protein molecules. In particular, allosterics, phosphoregulation, and spontaneous conversions (serpins). -

Science & Policy Meeting Jennifer Lippincott-Schwartz Science in The
SUMMER 2014 ISSUE 27 encounters page 9 Science in the desert EMBO | EMBL Anniversary Science & Policy Meeting pageS 2 – 3 ANNIVERSARY TH page 8 Interview Jennifer E M B O 50 Lippincott-Schwartz H ©NI Membership expansion EMBO News New funding for senior postdoctoral In perspective Georgina Ferry’s enlarges its membership into evolution, researchers. EMBO Advanced Fellowships book tells the story of the growth and ecology and neurosciences on the offer an additional two years of financial expansion of EMBO since 1964. occasion of its 50th anniversary. support to former and current EMBO Fellows. PAGES 4 – 6 PAGE 11 PAGES 16 www.embo.org HIGHLIGHTS FROM THE EMBO|EMBL ANNIVERSARY SCIENCE AND POLICY MEETING transmissible cancer: the Tasmanian devil facial Science meets policy and politics tumour disease and the canine transmissible venereal tumour. After a ceremony to unveil the 2014 marks the 50th anniversary of EMBO, the 45th anniversary of the ScienceTree (see box), an oak tree planted in soil European Molecular Biology Conference (EMBC), the organization of obtained from countries throughout the European member states who fund EMBO, and the 40th anniversary of the European Union to symbolize the importance of European integration, representatives from the govern- Molecular Biology Laboratory (EMBL). EMBO, EMBC, and EMBL recently ments of France, Luxembourg, Malta, Spain combined their efforts to put together a joint event at the EMBL Advanced and Switzerland took part in a panel discussion Training Centre in Heidelberg, Germany, on 2 and 3 July 2014. The moderated by Marja Makarow, Vice President for Research of the Academy of Finland. -

Carbohydrate Active Enzymes in Medicine and Biotechnology
19–21 AUGUST 2015 University of St Andrews, UK A joint Biochemical Society/ DEADLINES Royal Society of Chemistry Focused Meeting Abstract submission: Carbohydrate Active 15 JUNE 2015 Earlybird registration: Enzymes in Medicine 17 JULY 2015 and Biotechnology Organizers: Tracey Gloster Rob Field Gideon Davies Jerry Turnbull Overview: Carbohydrate active enzymes are vital in an Image kindly supplied by Tracey Gloster, University of St Andrews, UK Andrews, of St University Gloster, Tracey Image kindly supplied by abundance of cellular processes. These enzymes catalyse biologically important reactions and malfunction of these is often implicated in diseases. Fundamental to carbohydrate manipulation is gaining an understanding of such enzymes from a mechanistic, bioengineering, structural, functional, and biological viewpoint. Topics: * Insights into carbohydrate active enzymes in medicine * Use of carbohydrate active enzymes in biotechnology * Understanding mechanism and structure of carbohydrate active enzymes * Exploiting carbohydrate active enzymes in biosynthesis For a full programme please visit: www.biochemistry.org Sponsored by: 19–21 AUGUST 2015 University of St Andrews, UK A joint Biochemical Society/ Royal Society of Chemistry Focused Meeting DEADLINES Abstract submission: Carbohydrate Active 15 JUNE 2015 Enzymes in Medicine Earlybird registration: and Biotechnology 17 JULY 2015 Researching? Oral communication slots available. Award Lecture Studying? Apply for a student Sabine Flitsch – RSC Interdisciplinary Prize 2014 bursary online. -

Communicating Biochemistry: Meetings and Events
© The Authors. Volume compilation © 2011 Portland Press Limited Chapter 3 Communicating Biochemistry: Meetings and Events Ian Dransfield and Brian Beechey Scientific conferences organized by the Biochemical Society represent a key facet of activity throughout the Society’s history and remain central to the present mission of promoting the advancement of molecular biosciences. Importantly, scientific conferences are an important means of communicating research findings, establishing collaborations and, critically, a means of cementing the community of biochemical scientists together. However, in the past 25 years, we have seen major changes to the way in which science is communicated and also in the way that scientists interact and establish collabo- rations. For example, the ability to show videos, “fly through” molecular structures or show time-lapse or real-time movies of molecular events within cells has had a very positive impact on conveying difficult concepts in presentations. However, increased pressures on researchers to obtain/maintain funding can mean that there is a general reluctance to present novel, unpublished data. In addition, the development of email and electronic access to scientific journals has dramatically altered the potential for communi- cation and accessibility of information, perhaps reducing the necessity of attending meetings to make new contacts and to hear exciting new science. The Biochemical Society has responded to these challenges by progressive development of the meetings format to better match the -
Crystallography in the News New Product Introduction: Rigaku
Protein Crystallography Newsletter Volume 6, No. 5, May 2014 Crystallography in the news In this issue: May 3, 2014. A research team led by Professor Jennifer Potts, a British Heart Foundation Senior Research Fellow in York's Department of Biology, studied how Staphylococcus Crystallography in the news aureus attach to two proteins fibronectin and fibrinogen found in human blood. News: IUCr 23rd Congress May 4, 2014. Arizona State University scientists, together with collaborators from the Science video of the month Chinese Academy of Sciences in Shanghai, have published a first of its kind atomic level Upcoming events look at the enzyme telomerase that may unlock the secrets to the fountain of youth. Product spotlight: BioSAXS-2000 Crystallographers in the news May 7, 2014. Soon the Advanced Photon Source (APS) will get a boost in efficiency that likely will translate into a big boon for the discovery of new pharmaceuticals. The world's Scientist spotlight: Susan Buchanan first protein characterization research facility directly attached to a light source will open Useful links for crystallography in the near future at the APS. The Advanced Protein Characterization Facility (APCF) will Survey of the month use state-of-the-art robotics for gene cloning, protein expression, protein purification and protein crystallization. Recent crystallographic papers Book reviews May 12, 2014. Google honors Dorothy Hodgkin's X-ray vision with a molecular doodle. The event marks the 104th anniversary of the British chemist, who pioneered the use of X- rays to determine the structure of biological molecules. Special News Item IUCr 23rd Congress May 13, 2014. -
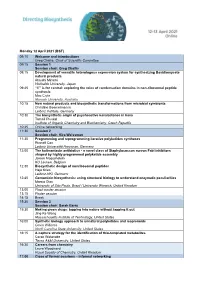
Monday 12 April 2021 (BST) 09:10 Welcome and Introductions Greg
Monday 12 April 2021 (BST) 09:10 Welcome and introductions Greg Challis, Chair of Scientific Committee 09:15 Session 1 Session chair: Greg Challis 09:15 Development of versatile heterologous expression system for synthesizing Basidiomycete natural products Atsushi Minami Hokkaido University, Japan 09:45 “C” is for central: exploring the roles of condensation domains in non-ribosomal peptide synthesis Max Cryle Monash University, Australia 10:15 New natural products and biosynthetic transformations from microbial symbionts Christine Beemelmanns Leibniz Institute, Germany 10:30 The biosynthetic origin of psychoactive kavalactones in kava Tomáš Pluskal Institute of Organic Chemistry and Biochemistry, Czech Republic 10:45 Online networking 11:30 Session 2 Session chair: Kira Weissman 11:30 Programming and reprogramming iterative polyketides synthases Russell Cox Leibniz Universität Hannover, Germany 12:00 The kalimantacin antibiotics - a novel class of Staphylococcus aureus FabI inhibitors shaped by highly programmed polyketide assembly Joleen Masschelein KU Leuven, Belgium 12:30 Biosynthetic design of nonribosomal peptides Hajo Kries Leibniz-HKI, Germany 12:45 Gentamicin biosynthesis: using structural biology to understand enzymatic peculiarities Marcio Dias University of São Paulo, Brazil / University Warwick, United Kingdom 13:00 Flash poster session 13:15 Poster session 14:15 Break 15:30 Session 3 Session chair: Sarah Barry 15:30 Making green drugs: tapping into nature without tapping it out Jing-Ke Weng Massachusetts Institute of Technology, -

Structural and Functional Studies of the Heptose Modifying Enzymes That Play a Role in Campylobacter Jejuni Virulence
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 1-15-2016 12:00 AM Structural and functional studies of the heptose modifying enzymes that play a role in Campylobacter jejuni virulence. Heba Soliman Barnawi The University of Western Ontario Supervisor Dr. Carole Creuzenet The University of Western Ontario Graduate Program in Microbiology and Immunology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Heba Soliman Barnawi 2016 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Microbiology Commons, Molecular Biology Commons, and the Structural Biology Commons Recommended Citation Barnawi, Heba Soliman, "Structural and functional studies of the heptose modifying enzymes that play a role in Campylobacter jejuni virulence." (2016). Electronic Thesis and Dissertation Repository. 3478. https://ir.lib.uwo.ca/etd/3478 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. ABSTRACT Campylobacter jejuni is a major cause of gastroenteritis in humans. The capsule of some species contains unique modified heptoses. Heptose modification and novel epimerases and reductases were identified for C. jejuni NCTC11168 and 18-176. We hypothesized that heptose modifying enzymes in C. jejuni have specific catalytic residues that allow for substrate and product specificity. Substrate synthesis, structural modeling, point mutations, and enzymatic analysis have been applied to map the active sites. Putative catalytic residues showed substrate and/or product specificity. -

Acknowledgment of Reviewers, 2017
Acknowledgment of Reviewers, 2017 The PNAS editors would like to thank all the individuals who dedicated their considerable time and expertise to the journal by serving as reviewers in 2017. Their generous contribution is deeply appreciated. A Katarzyna P. Adamala Hiroji Aiba Christine Alewine Jean Alric Hillie Aaldering Andrew Adamatzky Erez Lieberman Aiden Caroline M. Alexander Eben Alsberg Lauri A. Aaltonen Jozef Adamcik Allison E. Aiello R. Todd Alexander Manfred Alsheimer Duur K. Aanen Igor Adameyko Iannis Aifantis Alfredo Alexander-Katz Grégoire Altan-Bonnet Matthew L. Aardema Vic Adamowicz Christopher Aiken Anastassia N. Lee Altenberg Dirk G. A. L. Aarts David J. Adams Roger D. Aines Alexandrova Galit Alter Adam R. Abate David J. Adams Tracy D. Ainsworth Frank Alexis Orly Alter Cory Abate-Shen Jonathan Adams Edoardo Airoldi Emil Alexov Florian Altermatt Peter Abbamonte Michael E. Adams Jacqueline Michael Alfaro Marcus Altfeld Abul K. Abbas Patti Adank Aitkenhead-Peterson Hashim M. Al-Hashimi Dario C. Altieri Emmanuel A. Abbe R. Alison Adcock Slimane Ait-Si-Ali Asfar Ali Katye E. Altieri Shahal Abbo Zach N. Adelman Joanna Aizenberg Nageeb Ali Amnon Altman David H. Abbott Robert S. Adelstein Javier Aizpurua Robin R. Ali John D. Altman Geoffrey W. Abbott Andrew Adey Jaffer A. Ajani Saleem H. Ali Steven Altschuler Nicholas L. Abbott W. Neil Adger Caroline M. Ajo-Franklin Karen Alim Andrea Alu Omar Abdel-Wahab Jess F. Adkins Myles Akabas Michael T. Alkire Veronica A. Alvarez Ibrokhim Y. Ralph Adolphs Michael Akam Suvarna Alladi Javier Álvarez Abdurakhmonov Markus Aebi Omar S. Akbari Frédéric H.-T. Allain Arturo Alvarez-Buylla Ikuro Abe Yehuda Afek Erol Akcay David Alland David Amabilino Junichi Abe Hagit P. -

Chemical Engineering Explained
Advance Book Information February 2018 Chemical Engineering Chemical Engineering Explained Basic Concepts for Novices Basic Concepts for Chemical Engineering Explained Basic Concepts for Explained Novices David Shallcross Basic Concepts for Novices David Shallcross University of Melbourne, Australia Synopsis Shallcross Written for those less comfortable with science and mathematics, this text introduces the major chemical engineering topics for non-chemical engineers. With a focus on the practical rather than the theoretical, the reader will obtain a foundation in chemical engineering that can be applied directly to the workplace. By the end of this book, the user will be aware of the major considerations required to safely and efficiently design and operate a chemical processing facility. Case studies are included throughout, building a real-world connection. This book is ideal for professionals working with All information is subject to change without notice chemical engineers, and decision makers in chemical engineering industries. Brief Contents ISSN: Not Applicable l Introducing Processes Publisher: Royal Society of Chemistry l Working With Units ISBN: 9781782628613 Price: £59.99 | $95.99 l Chemistry Basics Publishing date: 01/02/2018 l Material and Energy Balances Target Audience: College/higher education l Phase Behaviour Format: Hardback Edition: 1 l Heat and Mass Transport Size: 234 x 156 l Fluid Mechanics Pages: 450 l Reaction Engineering BIC: PDG, TDCB l Separation Processes l Processes Instrumentation and Control To order -

Membership of Sectional Committees 2015
Membership of Sectional Committees 2015 The main responsibility of the Sectional Committees is to select a short list of candidates for consideration by Council for election to the Fellowship. The Committees meet twice a year, in January and March. SECTIONAL COMMITTEE 1 [1963] SECTIONAL COMMITTEE 3 [1963] Mathematics Chemistry Chair: Professor Keith Ball Chair: Professor Anthony Stace Members: Members: Professor Philip Candelas Professor Varinder Aggarwal Professor Ben Green Professor Harry Anderson Professor John Hinch Professor Steven Armes Professor Christopher Hull Professor Paul Attfield Professor Richard Kerswell Professor Shankar Balasubramanian Professor Chandrashekhar Khare Professor Philip Bartlett Professor Steffen Lauritzen Professor Geoffrey Cloke Professor David MacKay Professor Peter Edwards Professor Robert MacKay Professor Malcolm Levitt Professor James McKernan Professor John Maier Professor Michael Paterson Professor Stephen Mann Professor Mary Rees Professor David Manolopoulos Professor John Toland Professor Paul O’Brien Professor Srinivasa Varadhan Professor David Parker Professor Alex Wilkie Professor Stephen Withers SECTIONAL COMMITTEE 2 [1963] SECTIONAL COMMITTEE 4 [1990] Astronomy and physics Engineering Chair: Professor Simon White Chair: Professor Hywel Thomas Members: Members: Professor Girish Agarwal Professor Ross Anderson Professor Michael Coey Professor Alan Bundy Professor Jack Connor Professor Michael Burdekin Professor Laurence Eaves Professor Russell Cowburn Professor Nigel Glover Professor John Crowcroft -

AMP Binding Stabilizes the KTN Domain of the Shewanella
Subscriber access provided by Library, Special Collections and Museums, University of Aberdeen Article AMP binding stabilizes the KTN domain of the Shewanella denitrificans Kef potassium efflux system Christos Pliotas, Samuel C Grayer, Silvia Ekkerman, Anthony KN Chan, Jess Healy, Phedra Marius, Wendy Bartlett, Amjad Khan, wilian augusto cortopassi, Shane A Chandler, Tim Rasmussen, Justin LP Benesch, Robert S. Paton, Timothy D. W. Claridge, Samantha Miller, Ian Rylance Booth, James H. Naismith, and Stuart J. Conway Biochemistry, Just Accepted Manuscript • DOI: 10.1021/acs.biochem.7b00300 • Publication Date (Web): 28 Jun 2017 Downloaded from http://pubs.acs.org on June 28, 2017 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a free service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are accessible to all readers and citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. -

The Rhizoferrin Biosynthetic Gene in the Fungal Pathogen Rhizopus
Accepted Manuscript Title: The rhizoferrin <!–<query id="Q1">Please check the DOC¨ headfor¨ correctness.</query>–>biosynthetic gene in the fungal pathogen Rhizopus delemar is a novel member of the NIS gene family Authors: Cassandra S. Carroll, Clark L. Grieve, Indu Murugathasan, Andrew J. Bennet, Clarissa M. Czekster, Huanting Lui, James Naismith, Margo M. Moore PII: S1357-2725(17)30135-8 DOI: http://dx.doi.org/doi:10.1016/j.biocel.2017.06.005 Reference: BC 5153 To appear in: The International Journal of Biochemistry & Cell Biology Received date: 15-4-2017 Revised date: 30-5-2017 Accepted date: 3-6-2017 Please cite this article as: Carroll, Cassandra S., Grieve, Clark L., Murugathasan, Indu., Bennet, Andrew J., Czekster, Clarissa M., Lui, Huanting., Naismith, James., & Moore, Margo M., The rhizoferrin biosynthetic gene in the fungal pathogen Rhizopus delemar is a novel member of the NIS gene family.International Journal of Biochemistry and Cell Biology http://dx.doi.org/10.1016/j.biocel.2017.06.005 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. The rhizoferrin biosynthetic gene in the fungal pathogen Rhizopus delemar is a novel member of the NIS gene family Cassandra S.