Geographic Variation and Genetic Structure in the Bahama Oriole (Icterus Northropi), a Critically Endangered Synanthropic Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocalization Behavior of the Endangered Bahama Oriole (Icterus Northropi): Ontogenetic, Sexual, Temporal, Duetting Pair, and Geographic Variation Valerie A
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 3-1-2011 Vocalization Behavior of the Endangered Bahama Oriole (Icterus northropi): Ontogenetic, Sexual, Temporal, Duetting Pair, and Geographic Variation Valerie A. Lee Loma Linda University Follow this and additional works at: http://scholarsrepository.llu.edu/etd Part of the Biology Commons Recommended Citation Lee, Valerie A., "Vocalization Behavior of the Endangered Bahama Oriole (Icterus northropi): Ontogenetic, Sexual, Temporal, Duetting Pair, and Geographic Variation" (2011). Loma Linda University Electronic Theses, Dissertations & Projects. 37. http://scholarsrepository.llu.edu/etd/37 This Thesis is brought to you for free and open access by TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. It has been accepted for inclusion in Loma Linda University Electronic Theses, Dissertations & Projects by an authorized administrator of TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. For more information, please contact [email protected]. LOMA LINDA UNIVERSITY School of Science and Technology in conjunction with the Faculty of Graduate Studies ____________________ Vocalization Behavior of the Endangered Bahama Oriole (Icterus northropi): Ontogenetic, Sexual, Temporal, Duetting Pair, and Geographic Variation by Valerie A. Lee ____________________ A Thesis submitted in partial satisfaction of the requirements for the degree of Master of Science in Biology ____________________ March 2011 © 2011 Valerie A. Lee All Rights Reserved Each person whose signature appears below certifies that this thesis in his/her opinion is adequate, in scope and quality, as a thesis for the degree Master of Science. , Chairperson William K. Hayes, Professor of Biology Stephen G. -

A Comprehensive Species-Level Molecular Phylogeny of the New World
YMPEV 4758 No. of Pages 19, Model 5G 2 December 2013 Molecular Phylogenetics and Evolution xxx (2013) xxx–xxx 1 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev 5 6 3 A comprehensive species-level molecular phylogeny of the New World 4 blackbirds (Icteridae) a,⇑ a a b c d 7 Q1 Alexis F.L.A. Powell , F. Keith Barker , Scott M. Lanyon , Kevin J. Burns , John Klicka , Irby J. Lovette 8 a Department of Ecology, Evolution and Behavior, and Bell Museum of Natural History, University of Minnesota, 100 Ecology Building, 1987 Upper Buford Circle, St. Paul, MN 9 55108, USA 10 b Department of Biology, San Diego State University, San Diego, CA 92182, USA 11 c Barrick Museum of Natural History, University of Nevada, Las Vegas, NV 89154, USA 12 d Fuller Evolutionary Biology Program, Cornell Lab of Ornithology, Cornell University, 159 Sapsucker Woods Road, Ithaca, NY 14950, USA 1314 15 article info abstract 3117 18 Article history: The New World blackbirds (Icteridae) are among the best known songbirds, serving as a model clade in 32 19 Received 5 June 2013 comparative studies of morphological, ecological, and behavioral trait evolution. Despite wide interest in 33 20 Revised 11 November 2013 the group, as yet no analysis of blackbird relationships has achieved comprehensive species-level sam- 34 21 Accepted 18 November 2013 pling or found robust support for most intergeneric relationships. Using mitochondrial gene sequences 35 22 Available online xxxx from all 108 currently recognized species and six additional distinct lineages, together with strategic 36 sampling of four nuclear loci and whole mitochondrial genomes, we were able to resolve most relation- 37 23 Keywords: ships with high confidence. -

The Following Reviews Express the Opinions of the Individual Reviewers Regarding the Strengths, Weaknesses, and Value of The
REVIEWS EDITED BY M. ROSS LEIN Thefollowing reviews express the opinions of theindividual reviewers regarding the strengths, weaknesses, and value of thebooks they review. As such,they are subjective evaluations and do not necessarily reflect the opinions of theeditors or any officialpolicy of theA.O.U.--Eds. The Florida Scrub Jay: demographyof a cooper- geneticparents in the care of young that are not off- ative-breeding bird.--Glen E. Woolfenden and John spring of the helpers.To establishthat an individual W. Fitzpatrick. 1984. Princeton, New Jersey,Prince- is a helper, one must observeit caring for young that ton University Press. xiv + 406 pp., 1 color plate, are known not to be its own. There is no evidence many figures.ISBN 0-691-08366-5(cloth), 0-691-08367-3 in the chapter on procedures that Woolfenden and (paper).Cloth, $45.00;paper, $14.50.--For nearly two Fitzpatrick employed this criterion, although they decades three long-term studies of communally were aware of it (p. 4). Instead, throughout the book breeding birds, well known to readersof this review, jays are divided into breedersand helpers, implying have been in progress.The behavioral ecology of that if a bird is not a breeder it must be a helper these speciesis so complex that conclusionsreached ("Helpers are nonbreeders," p. 80). It is well known after only a few years of study can be quite mislead- for other speciesthat not all nonbreeders help, and ing. Eachnew year of studynot only enlargessample that individual nonbreedersvary significantlyin the sizesbut also provides insights that require impor- amount or intensity of their helping efforts. -

Can Urban Conservation Save a Critically Endangered Bird?
BIRDCONSERVATION The Magazine of American Bird Conservancy FALL 2017 BIRD’S EYE VIEW ABC’s New President: ABC is the Western Hemisphere’s bird conservation Putting Hope Into Action specialist—the only organization with a single and steadfast t is an enormous honor for me to take on the species are among the most heavily impacted. ABC commitment to achieving conservation results for native role of ABC President following in the footsteps will work with partners through BirdScapes and other Fall 2017 Iof George Fenwick. As we worked together for conservation tools to ensure that these birds and wild birds and their habitats more than 20 years, George and I collaborated with their habitats are adequately conserved. And we will throughout the Americas. the staff to develop American remain at the forefront to find BIRDCONSERVATION Bird Conservancy’s conservation both political and technological strategy. It has produced solutions to the main threats to A copy of the current financial statement and registration filed by the organization demonstrable results year after birds. may be obtained by contacting: ABC, P.O. 10 Airspace May Be the Final year, and I believe ABC is firmly Box 249, The Plains, VA 20198. 540-253- 5780, or by contacting the following state We will continue to conduct Ecological Frontier on the right track. agencies: our work with authenticity, Florida: Division of Consumer Services, I love birds and birding, and I want transparency, and dignity; we will toll-free number within the state: my children, and eventually their approach our partners thoughtfully 800-435-7352. 14 Can Urban Conservation children, to have the opportunity and with respect, even if we Maryland: For the cost of copies and postage: Office of the Secretary of State, Save a Rare Bird? to enjoy birds as I have. -

Bahamas: Endemics & Kirtland's Warbler 2019 BIRDS
Field Guides Tour Report Bahamas: Endemics & Kirtland's Warbler 2019 Mar 23, 2019 to Mar 27, 2019 Jesse Fagan For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Here we are in the Nassau airport at the end of our fun trip through the Bahamas. Thanks again to the group for a great time. This was another successful running of our short and fun itinerary to the Bahamas. We had awesome weather this year, and the birds didn't disappoint. We got to see all the possible endemics (including the local Bahama Oriole), several regional endemics (amazing looks at Great Lizard-Cuckoo and very cooperative West Indian Woodpecker), and, of course, wintering Kirtland's Warbler. Thanks to my fun group, and I look forward to seeing you again on another adventure. Jesse aka Motmot (from Dahlonega, Georgia) KEYS FOR THIS LIST One of the following keys may be shown in brackets for individual species as appropriate: * = heard only, I = introduced, E = endemic, N = nesting, a = austral migrant, b = boreal migrant BIRDS Podicipedidae (Grebes) LEAST GREBE (Tachybaptus dominicus) – A group of three birds seen on Eleuthera Island. PIEDBILLED GREBE (Podilymbus podiceps) – One distant bird seen on a large freshwater lake on Eleuthera Island. Columbidae (Pigeons and Doves) ROCK PIGEON (Columba livia) – In Marsh Harbour, Abaco Island. WHITECROWNED PIGEON (Patagioenas leucocephala) – Seen on all the islands. EURASIAN COLLAREDDOVE (Streptopelia decaocto) – This is where the North American invasion began. A burgled pet shop in 1974 and subsequent invasion of SE Florida was how it all started. -

Bahamasyou’Ve Heard of Our White Sand Beaches
DISCOVER THE BIRDS OF THE BahamasYou’ve heard of our white sand beaches. Our turquoise ocean waters. But what about our spectacular birds found nowhere else in the world? The Bahamas is a sublime spot for birdwatching the whole family will love. Beauty on a wing. The vibrant birds of The Bahamas will delight you. Search for over 300 species - including 6 species found only here - in landscapes of jaw-dropping natural beauty. Ocean adventures. Take a break from watching our colorful winged residents to watch our colorful aquatic ones. Grab a mask and explore coral reefs in some of the clearest waters in the world. Or use a rod and fly to find silvery bonefish in the turquoise blue. A chance to chill. After your outdoor exploration it’s time to put your feet up and relax. And there’s no better place to sip some sunshine and let the worries of the world fall away. Pack a beach umbrella and find your slice of heaven. Fun for the whole brood. Your choice for travel needs to bring smiles to the entire family. Luckily there’s something for everyone in The Bahamas Bring your brood to explore our islands and you won’t disappoint. Maximize your birding experience with an Audubon- trained guide. Want to see more of The Bahamas’ famed birds? Embark on your adventure with a local bird guide. Trained by Bahamas National Trust & Audubon experts, our bird guides have intimate knowledge of local birds and the best locations for spotting them. Hiring a guide also helps strengthen the local eco-economy, improving livelihoods while providing incentives for communities to protect important Cuban Emerald bird habitat. -

Bahamas: Abaco, Eleuthera, Andros and the Kirtland's Warbler 2015
Field Guides Tour Report Bahamas: Abaco, Eleuthera, Andros and the Kirtland's Warbler 2015 Apr 4, 2014 to Apr 9, 2015 Jesse Fagan For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Bahama Woodstar (Photo by participant Doug Hanna) This was another enjoyable island-hopping adventure with a fun group, excellent birding, and delicious food. It all started on the island of Abaco with a visit to Abaco Island National Park and our first Cuban Parrots perched in the pine trees and a Bahama Yellowthroat skulking in the understory. Bahama Palm Shores was also very good for parrots and West Indian Woodpecker, but lunch at Pete's Pub and "sittin' on the dock of the bay" in Cherokee Sound were also memorable. On to Eleuthera via our trusty Caravan, and within an hour we were looking at a pair of Kirtland's Warblers and our first Great Lizard-Cuckoo -- not too bad. But, again, lunch at Tippy's looking at quite possibly the prettiest beach in the world may have stolen the show, though we really enjoyed watching the sunset at The Cove! This was also the first year we offered a visit to Andros in search of the Bahama Oriole, a recent split from Greater Antillean Oriole. That turned out to be no problem, and we found its nest in the process. Turns out our group thought the Great Lizard-Cuckoo was the coolest thing since sliced bread, and I agree. It took home top honors, but it was in good company with Bahama Woodstar (female on the nest or maybe the male on Eleuthera?), and those parrots. -

Grouping Order Family Scientific Name Common
Complete bird listing for the Bahamas Grouping Order Family Scientific name Common Name Anis and allies Cuculiformes Cuculidae Coccyzus merlini Great Lizard Cuckoo Cuculiformes Cuculidae Coccyzus minor Mangrove Cuckoo Cuculiformes Cuculidae Crotophaga ani Smooth‐billed Ani Birds of prey Accipitriformes Accipitridae Accipiter striatus Sharp‐shinned Hawk Accipitriformes Accipitridae Buteo jamaicensis Red‐tailed Hawk Accipitriformes Accipitridae Circus cyaneus Northern Harrier Accipitriformes Accipitridae Elanoides forficatus American Swallow‐tailed Kite Accipitriformes Cathartidae Cathartes aura Turkey Vulture Accipitriformes Pandionidae Pandion haliaetus Osprey Accipitriformes Pandionidae Pandion haliaetus Osprey Falconiformes Falconidae Falco columbarius Merlin Falconiformes Falconidae Falco peregrinus Peregrine Falcon Falconiformes Falconidae Falco sparverius American Kestrel Strigiformes Strigidae Athene cunicularia Burrowing Owl Strigiformes Tytonidae Tyto alba Barn Owl Flamingos Phoenicopteriformes Phoenicopteridae Phoenicopterus ruber West Indian Flamingo Frigate birds and allies Suliformes Fregatidae Fregata magnificens Magnificent Frigate Bird Suliformes Phalacrocoracidae Phalacrocorax auritus Double‐crested Cormorant Suliformes Phalacrocoracidae Phalacrocorax brasilianus Neotropic Cormorant Suliformes Sulidae Morus bassanus Northern Gannet Suliformes Sulidae Sula dactylatra Suliformes Sulidae Sula leucogaster Brown Booby Suliformes Sulidae Sula sula Red‐footed Booby Game birds Galliformes Odontophoridae Colinus virginianus Northern -

Abstracts of Those Ar- Dana L Moseley Ticles Using Packages Tm and Topicmodels in R to Ex- Graham E Derryberry Tract Common Words and Trends
ABSTRACT BOOK Listed alphabetically by last name of presenting author Oral Presentations . 2 Lightning Talks . 161 Posters . 166 AOS 2018 Meeting 9-14 April 2018 ORAL PRESENTATIONS Combining citizen science with targeted monitoring we argue how the framework allows for effective large- for Gulf of Mexico tidal marsh birds scale inference and integration of multiple monitoring efforts. Scientists and decision-makers are interested Evan M Adams in a range of outcomes at the regional scale, includ- Mark S Woodrey ing estimates of population size and population trend Scott A Rush to answering questions about how management actions Robert J Cooper or ecological questions influence bird populations. The SDM framework supports these inferences in several In 2010, the Deepwater Horizon oil spill affected many ways by: (1) monitoring projects with synergistic ac- marsh birds in the Gulf of Mexico; yet, a lack of prior tivities ranging from using approved standardized pro- monitoring data made assessing impacts to these the tocols, flexible data sharing policies, and leveraging population impacts difficult. As a result, the Gulf of multiple project partners; (2) rigorous data collection Mexico Avian Monitoring Network (GoMAMN) was that make it possible to integrate multiple monitoring established, with one of its objectives being to max- projects; and (3) monitoring efforts that cover multiple imize the value of avian monitoring projects across priorities such that projects designed for status assess- the region. However, large scale assessments of these ment can also be useful for learning or describing re- species are often limited, tidal marsh habitat in this re- sponses to management activities. -
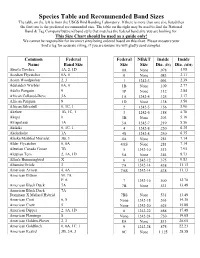
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American -

Population Status, Habitat Dependence, and Reproductive Ecology of Bahama Orioles: a Critically Endangered Synanthropic Species Melissa R
Journal of Field Ornithology J. Field Ornithol. 82(4):366–378, 2011 DOI: 10.1111/j.1557-9263.2011.00340.x Population status, habitat dependence, and reproductive ecology of Bahama Orioles: a critically endangered synanthropic species Melissa R. Price,1 Valerie A. Lee, and William K. Hayes Loma Linda University, Loma Linda, California 92354, USA Received 20 April 2011; accepted 29 July 2011 ABSTRACT. Recent elevation of critically endangered Bahama Orioles (Icterus northropi) to species status prompted us to evaluate their population status, habitat use, and breeding ecology. From surveys, we estimated that at least 141 to 254 individuals remain globally, with 90 to 162, 24 to 44, and 27 to 48 individuals remaining on North Andros Island, Mangrove Cay, and South Andros Island, The Bahamas, respectively. Orioles were observed nesting exclusively in anthropogenic habitat (residential and agricultural land), but home ranges also included nearby pine forest and coppice (dry broadleaf forest). Most nests (40 of 46, or 87%) were in nonnative coconut palm (Cocos nucifera), with native Sabal palmetto and Thrinax morrisii, and an introduced Brassaia actinophylla also used. Trees selected by orioles for nesting were significantly taller, less likely to have shrubs underneath, further from cover, and had more palm trees nearby than randomly selected palm trees. Three of eight nests with known contents were parasitized by Shiny Cowbirds (Molothrus bonariensis). Lethal yellowing disease recently devastated coconut palms and reduced the number of orioles on North Andros, but palms on Mangrove Cay and South Andros remain healthy. The juxtaposition of anthropogenic habitat to suitable native habitats may be more important than any single factor for Bahama Orioles, especially for breeding adults and fledged young. -

BIRDCONSERVATION the Magazine of American Bird Conservancy WINTER 2016-17 BIRD’S EYE VIEW
BIRDCONSERVATION The Magazine of American Bird Conservancy WINTER 2016-17 BIRD’S EYE VIEW The Nature of Threats to Birds ABC is the Western Hemisphere’s bird conservation specialist—the only organization ere’s a simple taxonomy of level effect” exists. What’s a few threats to birds. Those that birds lost to window collisions in with a single and steadfast Hare natural (normal preda- the larger scheme of things, these commitment to achieving tion, disease, weather events, etc.); scientists think. (In fact, it’s billions conservation results for native Winter 2016-17 those affecting reproduction (nest- each year.) We should put this wild birds and their habitats ing habitat loss or degradation); and myth to rest. What really matters is throughout the Americas. those affecting the survivorship of the cumulative effect of all threats BIRDCONSERVATION adults (everything else). How we on bird species’ populations— think about these in the context of especially the 40 percent or so A copy of the current financial statement human priorities is critical in under- that are now in decline. Such and registration filed by the organization Making a Safer World for Birds may be obtained by contacting: ABC, P.O. standing the future of birds. callousness for individual birds is Box 249, The Plains, VA 20198. 540-253- perilous in considering the future of 5780, or by contacting the following state We largely accept natural threats conservation. agencies: Wind Energy Takes On a New Look as an unfortunate matter of course. Bird populations have Florida: Division of Consumer Services, 10 Bird populations have evolved to Addressing threats to birds has been toll-free number within the state: 800-435-7352.