A-EA /A6m- July 2004 GOA UNIVERSITY Sub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SHIVAJI UNIVERSITY, KOLHAPUR Provisional Electoral Roll of Registered Graduates
SHIVAJI UNIVERSITY, KOLHAPUR Provisional Electoral Roll of Registered Graduates Polling Center : 1 Kolhapur District - Chh.Shahu Central Institute of Business Education & Research, Kolhapur Faculty - ARTS AND FINE ARTS Sr. No. Name and Address 1 ADAKE VASANT SAKKAPPA uchgaon kolhapur 416005, 2 ADNAIK DEVRAJ KRISHNAT s/o krishnat adnaik ,891,gaalwada ,yevluj,kolhapur., 3 ADNAIK DEVRAJ KRUSHANT Yevluj Panhala, 4 ADNAIK KRISHNAT SHANKAR A/P-KUDITRE,TAL-KARVEER, City- KUDITRE Tal - KARVEER Dist- KOLHAPUR Pin- 416204 5 AIWALE PRAVIN PRAKASH NEAR YASHWANT KILLA KAGAL TAL - KAGAL. DIST - KOLHAPUR PIN - 416216, 6 AJAGEKAR SEEMA SHANTARAM 35/36 Flat No.103, S J Park Apartment, B Ward Jawahar Nagar, Vishwkarma Hsg. Society, Kolhapur, 7 AJINKYA BHARAT MALI Swapnanjali Building Geetanjali Colony, Nigave, Karvir kolhapur, 8 AJREKAR AASHQIN GANI 709 C WARD BAGAWAN GALLI BINDU CHOUK KOLHAPUR., 9 AKULWAR NARAYAN MALLAYA R S NO. 514/4 E ward Shobha-Shanti Residency Kolhapur, 10 ALAVEKAR SONAL SURESH 2420/27 E ward Chavan Galli, Purv Pavellion Ground Shejari Kasb bavda, kolhapur, 11 ALWAD SANGEETA PRADEEP Plot No 1981/6 Surna E Ward Rajarampuri 9th Lane kolhapur, 12 AMANGI ROHIT RAVINDRA UJALAIWADI,KOLHAPUR, 13 AMBI SAVITA NAMDEV 2362 E WARD AMBE GALLI, KASABA BAWADA KOLHPAUR, 14 ANGAJ TEJASVINI TANAJI 591A/2 E word plot no1 Krushnad colony javal kasaba bavada, 15 ANURE SHABIR GUJBAR AP CHIKHALI,TAL KAGAL, City- CHIKALI Tal - KAGAL Dist- KOLHPUR Pin- 416235 16 APARADH DHANANJAY ASHOK E WARD, ULAPE GALLI, KASABA BAWADA, KOLHAPUR., 17 APUGADE RAJENDRA BAJARANG -
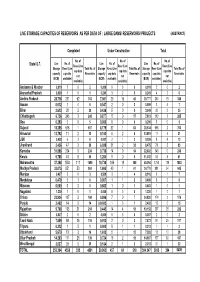
Live Storage Capacities of Reservoirs As Per Data of : Large Dams/ Reservoirs/ Projects (Abstract)
LIVE STORAGE CAPACITIES OF RESERVOIRS AS PER DATA OF : LARGE DAMS/ RESERVOIRS/ PROJECTS (ABSTRACT) Completed Under Construction Total No. of No. of No. of Live No. of Live No. of Live No. of State/ U.T. Resv (Live Resv (Live Resv (Live Storage Resv (Live Total No. of Storage Resv (Live Total No. of Storage Resv (Live Total No. of cap data cap data cap data capacity cap data Reservoirs capacity cap data Reservoirs capacity cap data Reservoirs not not not (BCM) available) (BCM) available) (BCM) available) available) available) available) Andaman & Nicobar 0.019 20 2 0.000 00 0 0.019 20 2 Arunachal Pradesh 0.000 10 1 0.241 32 5 0.241 42 6 Andhra Pradesh 28.716 251 62 313 7.061 29 16 45 35.777 280 78 358 Assam 0.012 14 5 0.547 20 2 0.559 34 7 Bihar 2.613 28 2 30 0.436 50 5 3.049 33 2 35 Chhattisgarh 6.736 245 3 248 0.877 17 0 17 7.613 262 3 265 Goa 0.290 50 5 0.000 00 0 0.290 50 5 Gujarat 18.355 616 1 617 8.179 82 1 83 26.534 698 2 700 Himachal 13.792 11 2 13 0.100 62 8 13.891 17 4 21 J&K 0.028 63 9 0.001 21 3 0.029 84 12 Jharkhand 2.436 47 3 50 6.039 31 2 33 8.475 78 5 83 Karnatka 31.896 234 0 234 0.736 14 0 14 32.632 248 0 248 Kerala 9.768 48 8 56 1.264 50 5 11.032 53 8 61 Maharashtra 37.358 1584 111 1695 10.736 169 19 188 48.094 1753 130 1883 Madhya Pradesh 33.075 851 53 904 1.695 40 1 41 34.770 891 54 945 Manipur 0.407 30 3 8.509 31 4 8.916 61 7 Meghalaya 0.479 51 6 0.007 11 2 0.486 62 8 Mizoram 0.000 00 0 0.663 10 1 0.663 10 1 Nagaland 1.220 10 1 0.000 00 0 1.220 10 1 Orissa 23.934 167 2 169 0.896 70 7 24.830 174 2 176 Punjab 2.402 14 -

Avifauna and Comparative Study of Threatened Birds at Urban Wetlands of Kolhapur, Maharashtra, India
Int. J. of Life Sciences, 2017, Vol. 5 (4): 649-660 ISSN: 2320-7817| eISSN: 2320-964X RESEARCH ARTICLE Avifauna and Comparative Study of Threatened Birds at Urban Wetlands of Kolhapur, Maharashtra, India Patil Nachiket Suryakant Department of Environment Science, Shivaji University, Kolhapur-416 004, Maharashtra, India Email: - [email protected] | (+918805835153) Manuscript details: ABSTRACT Received: 28. 10.2017 Kolhapur is blessed with numbers of wetlands which is productive and Accepted: 05.12.2017 unique ecosystem that support number of birds. The present study focus on Published : 31.12.2017 avifaunal diversity and population status of threatened bird species by Diversity indices at in and around the wetlands of Kolhapur from 2016- Editor: 2017. Bird survey was conducted according to line transect method and Dr. Arvind Chavhan standard point count method. Species Diversity indices and Simpson index were used for statistical analysis. A total of 159 species of birds belonging to Cite this article as: 17 orders and 60 families were recorded, of those 109 species of terrestrial Patil Nachiket Suryakant (2017) birds and 50 species of water birds were observed. Out of 159 birds, 93 Avifauna and Comparative Study of Threatened Birds at Urban Wetlands residential, 50 residential migratory and 15 are migratory. Of those one of Kolhapur, Maharashtra, India; species is vulnerable, three species near threatened were observed. Among International J. of Life Sciences, 5 (4): the birds recorded in study area, about 43% were insectivores and other 649-660. dominating types include mixed diet 10%, piscivores 15%, omnivores 9%, carnivores 9%, granivores 9%, fructivores 2% and nectivores 3% Acknowledgement respectively. -

09 Chapter 3.Pdf
CHAPTER ID IDENTIFICATION OF THE TOURIST SPOT 3.1The Kolhapur City 3.2 Geographical Location 3.3 History 3.4 Significance of Kolhapur for the Study [A] Aspects and Outlying belts [B] Hill top konkan and the plain [C] Hills [D] Rive [E] Ponds and lakesrs [F] Geology [G] Climate [H] Forests [I] Flora of Kolhapur District [J] Vegetation [K] Grassland [L] Economically important plants [P] Wild Animals [Q] Fishers 3.5 Places of Interest in the selected area and their Ecological Importance. 1. New Palace 2. Rankala Lake 3. The Shalini Palace 4. Town Hall 5. Shivaji University 6. Panctiaganga Ghat 7. Mahalaxmi Temple 8. Temblai Hill Temple Garden 9. Gangawesh Dudh Katta 3.6 Place of Interest around the Kolhapur / Selected area and their ecological importance. 1. Panhala Fort 2. Pawankhind and Masai pathar 3. Vishalgad 4. Gaganbavada / Gagangad 5. Shri Narsobachi Wadi 6. Khirdrapnr: Shri Kopeshwar t«pk 7. Wadi Ratnagh-i: Shri Jyotiba Tmepie 8. Shri BahobaM Temple 9. RaAaatgiii and Dajqror Forest Reserves 10. Dob wade falls 11. Barld Water Fails 12. Forts 13. Ramteeth: 14. Katyayani: 15 The Kaneri Math: 16 Amba Pass 3.7 misceieneoas information. CHAPTER -HI IDENTIFICATION OF THE TOURIST SPOT. The concept of Eco-Tourism means making as little environmental impact as possible and helping to sustain the indigenous populace thereby encouraging, the preservation of wild life and habitats when visiting a place. This is responsible form of tourism and tourism development, which encourages going back to natural products in every aspects of life. It is also the key to sustainable ecological development. -

List of Participants COMPLETE LIST of ALL QUALIFIED PARTICIPANTS with THEIR PROJECT NAMES
2008 landscape competition | List of Participants COMPLETE LIST OF ALL QUALIFIED PARTICIPANTS WITH THEIR PROJECT NAMES B.K.P.S. College of Architecture, Pune Bharati Vidyapeeth College of Architec- Guru Nanak Dev University, Amritsar 1. SHANIWAR WADA, PUNE ture, Pune 39. CENTRAL BUSINESS DISTRICT, NEW AMRITSAR Ajay Mane Ashish Batra, Karan Ganeriwala 21. RIVERFRONT DEVELOPMENT AT VITHALWADA, PUNE Faquih Museeb Talha, Pokale Parag Shriram 2. KASTURCHAND PARK, NAGPUR IIT, Rorkee Atreyee Ghosh 22. SEASONS - A GATHERING PLACE 40. COMMUNITY RECREATIONAL CENTRE, MUNDRA SEZ Kunal Deepchand Rathi, Prachi Satish Deshpande Abhijeet Sharma, Gaurav Mangla, Hrishi Raj Agarwalla 3. RIVERFRONT DEVELOPMENT FOR VITHALWADI, PUNE Neha Sanjeev Unawane 24. CULTURAL PORT OF COCHIN, TIDES OF CHANGE Jadhavpur University, Kolkata Parikh Ankit Anil, Neha Chavan 4. REVIVED IDENTITY 41. MAIDAN, KOLKATA Pradnya Rajan Nesarikar, Smita Yatish Prabhune, Roshmi Sen Sarang Sanjay Patwardhan Chitkara School of Architecture and Planning, Patiala Jamia Millia Islamia, New Delhi 5. PATALESHWAR CAVE COMPLEX 25. REVIIVING, NEHRU PLACE 42. COMMUNITY CENTRE, NEW FRIENDS COLONY Sneha Jagdish Thakur Hina Sahi, Kavita, Himanshu Suri Apurva Anand, Mansha Samreen Bharati Vidyapeeth College of Architec- College of Engineering, 43. NEHRU PLACE, LANDSCAPE Arshad Nafees, Kajoli Kerketta ture, Navi Mumbai Thiruvanathapuram 6. AMBE GHOSALE LAKE 26. BRIDGING THE HORIZON 44. SCAPING THE PRECINCT, JAMA MASJID Abhineet P. Ghag, Durgesh Tatkar, Vibhavari Patil Roshan Alexandar, Hetal J. Patel Iqtedar Alam, Ahmad Faraz Ansari 7. REVITALISING WELLINGTON CIRCLE 45. SARAI JULLENA PARK Amit Yadav, Suraj V. Majithia, Taher Rangwala CSIIT, Secundrabad 27. GATED COMMUNITY Kumar Abhishek, Md. Waseem Reza 8. GOLMAIDAN Koganti Ujwala 46. 127 ACRES NEAR NEHRU PLACE Asim Sadiq - Wadkar, Nilesh Bhojwani, Akshansh Md. -

Annexure-V State/Circle Wise List of Post Offices Modernised/Upgraded
State/Circle wise list of Post Offices modernised/upgraded for Automatic Teller Machine (ATM) Annexure-V Sl No. State/UT Circle Office Regional Office Divisional Office Name of Operational Post Office ATMs Pin 1 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA PRAKASAM Addanki SO 523201 2 Andhra Pradesh ANDHRA PRADESH KURNOOL KURNOOL Adoni H.O 518301 3 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM AMALAPURAM Amalapuram H.O 533201 4 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Anantapur H.O 515001 5 Andhra Pradesh ANDHRA PRADESH Vijayawada Machilipatnam Avanigadda H.O 521121 6 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA TENALI Bapatla H.O 522101 7 Andhra Pradesh ANDHRA PRADESH Vijayawada Bhimavaram Bhimavaram H.O 534201 8 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA VIJAYAWADA Buckinghampet H.O 520002 9 Andhra Pradesh ANDHRA PRADESH KURNOOL TIRUPATI Chandragiri H.O 517101 10 Andhra Pradesh ANDHRA PRADESH Vijayawada Prakasam Chirala H.O 523155 11 Andhra Pradesh ANDHRA PRADESH KURNOOL CHITTOOR Chittoor H.O 517001 12 Andhra Pradesh ANDHRA PRADESH KURNOOL CUDDAPAH Cuddapah H.O 516001 13 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM VISAKHAPATNAM Dabagardens S.O 530020 14 Andhra Pradesh ANDHRA PRADESH KURNOOL HINDUPUR Dharmavaram H.O 515671 15 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA ELURU Eluru H.O 534001 16 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudivada Gudivada H.O 521301 17 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudur Gudur H.O 524101 18 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Guntakal H.O 515801 19 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA -

Government of India Ministry of Jal Shakti, Department of Water Resources, River Development & Ganga Rejuvenation Lok Sabha Unstarred Question No
GOVERNMENT OF INDIA MINISTRY OF JAL SHAKTI, DEPARTMENT OF WATER RESOURCES, RIVER DEVELOPMENT & GANGA REJUVENATION LOK SABHA UNSTARRED QUESTION NO. †919 ANSWERED ON 27.06.2019 OLDER DAMS †919. SHRI HARISH DWIVEDI Will the Minister of JAL SHAKTI be pleased to state: (a) the number and names of dams older than ten years across the country, State-wise; (b) whether the Government has conducted any study regarding safety of dams; and (c) if so, the outcome thereof? ANSWER THE MINISTER OF STATE FOR JAL SHAKTI & SOCIAL JUSTICE AND EMPOWERMENT (SHRI RATTAN LAL KATARIA) (a) As per the data related to large dams maintained by Central Water Commission (CWC), there are 4968 large dams in the country which are older than 10 years. The State-wise list of such dams is enclosed as Annexure-I. (b) to (c) Safety of dams rests primarily with dam owners which are generally State Governments, Central and State power generating PSUs, municipalities and private companies etc. In order to supplement the efforts of the State Governments, Ministry of Jal Shakti, Department of Water Resources, River Development and Ganga Rejuvenation (DoWR,RD&GR) provides technical and financial assistance through various schemes and programmes such as Dam Rehabilitation and Improvement Programme (DRIP). DRIP, a World Bank funded Project was started in April 2012 and is scheduled to be completed in June, 2020. The project has rehabilitation provision for 223 dams located in seven States, namely Jharkhand, Karnataka, Kerala, Madhya Pradesh, Orissa, Tamil Nadu and Uttarakhand. The objectives of DRIP are : (i) Rehabilitation and Improvement of dams and associated appurtenances (ii) Dam Safety Institutional Strengthening (iii) Project Management Further, Government of India constituted a National Committee on Dam Safety (NCDS) in 1987 under the chairmanship of Chairman, CWC and representatives from State Governments with the objective to oversee dam safety activities in the country and suggest improvements to bring dam safety practices in line with the latest state-of-art consistent with Indian conditions. -

Tilak Maharashtra Vidyapeeth, Pune for the Degree of Doctor of Philosophy(Ph.D.) in Geography Subject Under the Board of Moral and Social Sciences Studies
SPATIO-TEMPORAL CHANGES OF POPULATION OF SANGLI DISTRICT A Thesis submitted to Tilak Maharashtra Vidyapeeth, Pune For the Degree of Doctor of Philosophy(Ph.D.) In Geography Subject Under the Board of Moral and Social Sciences Studies Submitted By Mr. D. B. KARNIK Under theGuidance of Dr. T. M. VARAT M.A., Ph.D. Head Department of Geography New Arts Commerce and Science College, Ahmednagar Feb.-2015 CERTIFICATE This is to certify that the thesis entitled, “SPATIO-TEMPORAL CHANGES OF POPULATION OF SANGLI DISTRICT”. Which is being submitted herewith for the award of the Degree of Vidyavachaspati (Ph. D.) in Geography of Tilak Maharashtra Vidyapeeth, Pune is the result of original research work completed by Mr. Dhananjay Bhimrao Karnik under my supervision and guidance. To the best of my knowledge and belief the work incorporated in this thesis has not formed the basis for the award of any Degree or similar title of this or any other University or examining body. Research Guide Date : Dr. T. M. VARAT Head Department of Geography, New Arts Commerce and Science College, Ahmednagar i DECLARATION I hereby declare that the thesis entitled “SPATIO-TEMPORAL CHANGES OF POPULATION OF SANGLI DISTRICT” is the original research work carried out by me under the guidance of Dr. T. M. Varat, Head, Department of Geography, New Arts Commerce and Science College, Ahmednagar for the award of Ph. D. degree in Geography to the Tilak Maharashtra Vidyapeeth, Pune. This has not been submitted previously for the award of any degree or diploma in any other university. Date : Mr. D. -

6. Water Quality ------61 6.1 Surface Water Quality Observations ------61 6.2 Ground Water Quality Observations ------62 7
Version 2.0 Krishna Basin Preface Optimal management of water resources is the necessity of time in the wake of development and growing need of population of India. The National Water Policy of India (2002) recognizes that development and management of water resources need to be governed by national perspectives in order to develop and conserve the scarce water resources in an integrated and environmentally sound basis. The policy emphasizes the need for effective management of water resources by intensifying research efforts in use of remote sensing technology and developing an information system. In this reference a Memorandum of Understanding (MoU) was signed on December 3, 2008 between the Central Water Commission (CWC) and National Remote Sensing Centre (NRSC), Indian Space Research Organisation (ISRO) to execute the project “Generation of Database and Implementation of Web enabled Water resources Information System in the Country” short named as India-WRIS WebGIS. India-WRIS WebGIS has been developed and is in public domain since December 2010 (www.india- wris.nrsc.gov.in). It provides a ‘Single Window solution’ for all water resources data and information in a standardized national GIS framework and allow users to search, access, visualize, understand and analyze comprehensive and contextual water resources data and information for planning, development and Integrated Water Resources Management (IWRM). Basin is recognized as the ideal and practical unit of water resources management because it allows the holistic understanding of upstream-downstream hydrological interactions and solutions for management for all competing sectors of water demand. The practice of basin planning has developed due to the changing demands on river systems and the changing conditions of rivers by human interventions. -

College / Organisation ROHANT SIDDHARAM DHABBE JCJ Sachinkumar R
Full name Name of the institute/ college / organisation ROHANT SIDDHARAM DHABBE JCJ Sachinkumar R. Patil Jaysingpur College Jaysingpur Sheetal Dattatray Londhe Willingdon college Sangli KRISHNA MAHAVIDYALAYA, RETHARE BK., SAMPATRAO SHIVAJIRAO PATIL KARAD Dr.Tushar Ganpatrao Ghatage Jaysingpur College, Jaysingpur Lata Parasharam Bhopale Rajaram college Kolhapur Jyoti Ashok Chavan Rajaram College Kolhapur Rajaram Santram Sawant Dr. Ghali College, Gadhinglaj Prof.Neeta Vijayrao Jagtap P.D.E.A's Waghire College sas Srichand Parsram Hinduja Sree Narayana Guru College of Commerce Dr Anil N Dadas Dahiwadi College SAHYADRI SHIKSHAN SEVA MANDAL'S ARTS AND Rima Deepak Gharat COMMERCE COLLEGE Payal Hinduja St. Paul Degree College MSS'S ARTS, SCIENCE & COMMERCE COLLEGE, SHARAD KHANDU KHOJE AMBAD DIST.JALNA Sakharam Damu Aghav B. G. College, Sangvi Pandhare Balasaheb Dashrath New Law College Ahmednagar Swapnil Vasantrao Sonje Dr.D.Y.Patil College of Physiotherapy, Pune Ajeet Singh Sanatan Dharma College, Ambala Cantt. Dadasaheb Dhanaji Nana Chaudhari Social Work College, Ravindra Amrut Patil Malkapur, Dist. Buldana Comrade Godavari Shamrao Parulekar College of Arts VILAS VASANT RAYMALE Commerce and Science Talasari Swati Sarwate St. Mira's college for girls Koregaon park Pune Swapneel Dipchand Agarwal Sadguru Gadage Maharaj College, Karad Sandhya Jaysing Mane Chandrabai Shantappa Shendure College Hupari Dattatraya Ramchandra Bhosale Chandrabai-Shantappa Shendure College, Hupari Pritam Raygonda Patil Jaysingpur College,Jaysingput. Sandip Jotiram Kirdat Chhatrapati Shivaji College Satara (Autonomous) Sarika Vishwas More Chandrabai Shanttappa Shendure College, Hupari Jayvant Balavant Patade Jaysingpur College, Jaysingpur Prajakta Pramod Rajmane Bharati Vidyapeeth college of Engineering ,Kolhapur Suraj Ashok Sonawane Rajaram College Kolhapur Aishwarya Prasad Otari S.M.Joshi College, Hadapsar Dr. -
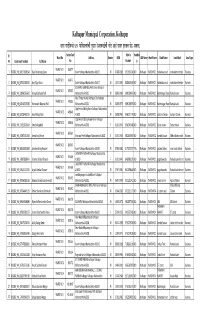
Street Vendor List Having Double Ration Card
Kolhapur Municipal Corporation, Kolhapur ºÉnù®ú ªÉÉnùÒ¨ÉvªÉä 492 ¡äò®Ò´ÉɱªÉÉÆSÉÒ nÖù¤ÉÉ®ú ®äú¶ÉxÉEòÉbÇ÷SÉÒ xÉÉånù +ɽäþ ªÉÉ´É®ú ½þ®úEòiÉ PÉä>ð ¶ÉEòiÉÉ. Sr. Ration Card Mobile Handica Ward No Address Gender DOB ULB Name Ward Name Road Name Land Mark Area Type no Enrollment Number Full Name No Number p WARD NO 1 454477 1 802887_HK_969171005548 Vijay Pandurang Gurav Karvir Kolhapur Maharashtra 416012 M 02-08-1962 9923913545 NO Kolhapur WARD NO 1 mahadava road mahalaxme temple Business WARD NO 1 454477 2 802887_HK_847532180392 Ajay Vijay Gurav Karvir Kolhapur Maharashtra 416012 M 14-11-1991 8605626464 NO Kolhapur WARD NO 1 mahadava road mahalaxme temple Business KOLHAPUR SAW MIL JAVAL Karvir Kolhapur WARD NO 1 473033 3 802887_HK_328960563610 Vinayak Baburao Patil Maharashtra 416012 M 08-06-1984 9890189976 NO Kolhapur WARD NO 1 Radhanagari Road Rankala Lake Business Near Timber market Kolhapur City Kolhapur WARD NO 1 473033 4 802887_HK_642400535583 Vishwanath Baburao Patil Maharashtra 416012 M 23-09-1979 9890189976 NO Kolhapur WARD NO 1 Radhanagari Road Rankala Lake Business Opp Perina Coldrig Karvir Kolhapur Maharashtra WARD NO 2 485948 5 802887_HK_875284463145 Amol Bhikaji Mali 416002 M 26-08-1991 9960717197 NO Kolhapur WARD NO 2 dashara Chowk dashara Chowk Business Opp Perina Coldrig House Karvir Kolhapur WARD NO 2 485948 6 802887_HK_731929629654 Uma Bhikaji Mali Maharashtra 416002 F 01-01-1947 9960745488 NO Kolhapur WARD NO 2 Dasra chowk Dasra chowk Business WARD NO 2 845760 7 802887_HK_590875311334 Amina Siraj Nurani Shaniwar Peth Kolhapur -

SHIVAJI UNIVERSITY, KOLHAPUR for the Election of Principals Of
SHIVAJI UNIVERSITY, KOLHAPUR Final Electoral Roll For the Election of Principals of Affiliated / Conducted / Autonomous Colleges to the Senate under section 28(2)(o) of the Maharashatra Public Universirties Act,2016 Polling Center : - 1 Kolhapur District - Chh.Shahu Central Institute of Business Education & Research, Kolhapur 1 MORE HARINATH NIVRUTTI Bharati Vidyapiths College Of Pharmacy,Kolhapur 2 ALI MOHAMMAD M. Chh. Shahu Central Institute Of Business Education& Research,Kolhapur 3 CHOUGALE PRAVEEN NARAYAN D.R. Mane Mahavidyalaya,Kagal 4 GURAV ASHISH KRISHANRAO Dhananjay Mahadik Group Of Institutions,Kolhapur 5 KARJINI VILAS VIJAY Kits College Of Engineering,Kolhapur 6 PATIL JAKKAPP BAPU Kamala College,Kolhapur 7 KHILARE CHANDRAKANT JAGANNATH Rajarshi Chh. Shahu College,Kolhapur 8 LAKADE SUNITA SURESH Shikshanshastra Mahavidyalaya,Kagal Page No.- 1 SHIVAJI UNIVERSITY, KOLHAPUR Final Electoral Roll For the Election of Principals of Affiliated / Conducted / Autonomous Colleges to the Senate under section 28(2)(o) of the Maharashatra Public Universirties Act,2016 Polling Center : - 2 Kolhapur District - Shri Shahaji Chh. Mahavidyalaya, Dasara Chowk, Kolhapur 9 SHAHA NANDKUMAR VIDYACHANDRA Anandi Arts, Commerce & Science College Gaganbavada,Kolhapur 10 PATIL DINKAR VISHNU Bhogavati Mahavidyalaya,Kurukali 11 KULAKRNI GIRIJA GIRISH Kala Prabodhini'S Institute Of Design,Kolhapur 12 LOKHANDE RAJENDRA PRABHAKAR Mahavir Mahavidyalaya,Kolhapur 13 MORUSKAR DHANAJI SHAMRAO Radhanagari Mahavidyalaya,Radhanagari 14 BHOSALE SARITA JALINDAR Shri S.K.