Efficient Propagation with in Vitro Seed Germination of Vanda Falcata*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SOOS November 2019
SOUTHERN ONTARIO ORCHID SOCIETY November 2019, Volume 54, Issue 10 Meeting since 1965 Next Meeting Sunday, November 3, Floral Hall of the Toronto Botanical Garden. Vendor sales noon to 1pm. Noon, Culture talks on the stage by Alexsi Antanaitis. Topic ? Program at 1pm Our guest speaker George Hatfield, owner and operator of Hatfield Orchids in, Oxnard, CA will speak on Cymbidiums. George is an AOS and Cymbidium Society judge and a hybridizer. Monthly show table. Bring your flowering plants for show and tell and points towards our annual awards. Raffle President’s Remarks Welcome Orchid Enthusiasts. Fall has come, although as I write this in mid-October, the temperatures in my area have Don will be on the lookout for plants, so please help not yet neared freezing, so my plants are still him out by sending some of your plants on a road outdoors. The cool nights are helping set buds on trip to Southwestern Ontario. They may even come my Phalaenopsis, and helping to “harden off” the back with some awards. summer growths of my Cattleyas, which should lead to a bountiful display of blooms over the next Thank you in advance for those members who few months. I’ve already moved a few plants with generously lend their precious plants. The SOOS buds indoors under lights, in order to speed up the displays could not happen without you. opening of the blooms. The rest of my plants will come indoors over the next 2 weeks (or faster if the Our future meetings for the remainder of this year are as weather necessitates, i.e frost warnings). -

(Neofinetia) Falcata: an Interview with Satomi Kasahara Phyllis Prestia
©Eric Hunt GROWING VANDA (NEOFINETIA) FALCATA: AN INTERVIEW WITH SATOMI KASAHARA PHYLLIS PRESTIA Neofinetia falcata HE SANTA BARBARA AREA is beautiful in Seed Engei the summer. Mild ocean breezes bathe the coast Shigeru Kasahara has been active in the Fūkiran So- under a warm California sun. For orchid lovers, T ciety in Japan for over 25 years and currently serves as it’s a paradise. July brings the annual open houses for the Chairman, an honored position. It was his friend’s two of the area orchid nurseries, Santa Barbara Orchid grandfather who gave him his first orchid, Dendrobium Estate and Cal-Orchid Inc. And for me, it brings an op- moniliforme and stirred his interest in native Japanese portunity to attend the meeting of the Fūkiran Society orchids. Shigeru loved plants, but Japanese orchids had of America and the annual Fūkiran judging, supported grabbed his heart. Eventually, Shigeru concentrated by the Japanese Fūkiran Society. For several years, the mostly on growing Fūkiran and sold them in his newly meeting and judging have been held on the property established flower shop. In 1983, he established Seed of Cal-Orchid Inc. on Saturday during the annual open Engei, a Japanese nursery which promotes and sells na- house weekend. This year they were held on July 14th, tive Japanese orchids and their hybrids. and this year I entered a few of my own Vanda falcata In the early 2000s, Shigeru visited the United States. orchids. He met Jason Fischer at his nursery, Orchids Limited The American Fūkiran Society was established in (Orchid Web.com) in Plymouth, Minnesota and the late 2012 as an offshoot of the Japan Fūkiran Society. -

Orchid Research Newsletter No. 66
Orchid Research Newsletter No. 66 When the Molecular Systematics Laboratory at the Royal Botanic Gardens, Kew, first opened under the leadership of Mark Chase and Mike Bennett as Keeper of the Jodrell, the main target marker was rbcL for family-wide systematic studies of Orchidaceae and other families. Internal transcribed spacers of nuclear ribosomal DNA were used for studies below the family level. Those of us unfortunate to have lived through those days of manual sequencing in the early 1990s know how laborious it was to label nucleotides with radioactive phosphorus 32 or sulfur 35 with all the safety measures that that entailed, pour polyacrylamide gels between two glass plates without even the smallest air bubble, expose the sequencing gel to x-ray film, and then manually call the bases one by one off the autoradiogram, hoping to get 100 bases of the more than 1300 base pairs in the rbcL sequence and then enter them into the computer database. In many cases, the results were ambiguous, which entailed judgment calls. And this did not even include data analysis with software much, much slower than today. All this work produced only a trickle of data and a lack of strong support for many branches leading to the major clades. Manna from heaven came in the form of automated sequencing later that decade. Automated sequencers used the Sanger method, which relied on the introduction of dideoxynucleotides into the growing DNA strand by DNA polymerase, creating fragments separated by size on the gel by electrophoresis. Nucleotides were labeled by fluorescent dyes rather than sulfur 35, and read by a laser. -
August 2014 Plant Table Awards June 4, 2014 First Place, a Yellow Ers on a Plant Hardy in Oncidium Hybrid Our Area
Manatee-Sarasota Group Boca Next Meeting: Wednesday A Message from your President: August 6th at 7:00 p.m. How many of you believe in the Speaker: Courtney Hackney karma of orchids? I know I do. It Topic: “Growing Cattleyas ” doesn’t matter how many plants Professor Hackney is the Director of you have; there are a few special Coastal Biology at the University of orchids that you love more than the North Florida in Jacksonville, Flori- others. The ones that speak to you da. He began growing orchids in the and always do better because you Florida Keys in 1962 while working somehow know when they need John Masters for a small orchid nursery and has something extra or special. continued his interest in both orchid This karma is also therapeutic. It opens your mind hybridizing and orchid culture since to another world that requires thinking, planning and then. He grows many different genera, caring. It also provides escape from the everyday worries but his favorite is the Cattleya Alliance. He has about 500 we all have. Let’s not discuss the obsessive behavior mature cattleyas and even more seedlings, but his favor- part of it; we all know this already. This new kind of ites are classic clones, some of which appeared in orchid thinking is relaxing also. My working life was consumed collections over 100 years ago. He makes 8-10 hybrids and by deductive reasoning. In my law enforcement world species sib crosses per year in various genera. A always led to B and then C and then someone was He wrote a Growing Tips column for 20 years, which in handcuffs. -

Southern Ontario Orchid Society News
SOUTHERN New to GoToMeeting? Get the app now and be ready when your first meeting starts: ONTARIO https://global.gotomeeting.com/install/733560421 Please note that because this meeting is being ORCHID produced via an internet program the participation capacity is limited to SOOS members only. Be sure to check that you have renewed your membership for SOCIETY NEWS 2020. 2020 Memberships have been extended to the April 2021, Volume 56, Issue 4 end of 2021. Meeting since 1965 April 4 Virtual Meeting: Virtual Show Table. Our members are growing and blooming amazing orchids and you are not shy about sharing photographs of them. Our virtual show table has doubled in size in less than six months. The March 7th Show Table was Dave Sorokowsky, Paph Paradise awesome. We had 105 submissions from 29 different members!! Paphiopedilum Culture We encourage you to group several plants in a small display th or basket. This group can include any number of plants and Sunday, April 4 , 2021, 1:00 PM (EDT) any combination of species, hybrids, and genera. Keep in mind that the slides are wide and short; about 10” x 6” (22 Followed by the cm x 13 cm). The names of the gorgeous flowers in the arrangement are still very important. SOOS Virtual Show Table This will allow us to show even more of your blooming Entry Rules: orchids. Looking forward to more great photos. Don’t forget to send yours. 1. Take photos between Sun. March st st 21 and Wed. March 31 . President’s Remarks Welcome Orchid Enthusiasts 2. -

178. NEOFINETIA Hu, Rhodora 27: 107. 1925. 风兰属 Feng Lan Shu Chen Xinqi (陈心启 Chen Sing-Chi); Jeffrey J
Flora of China 25: 483–484. 2009. 178. NEOFINETIA Hu, Rhodora 27: 107. 1925. 风兰属 feng lan shu Chen Xinqi (陈心启 Chen Sing-chi); Jeffrey J. Wood Finetia Schlechter, Beih. Bot. Centralbl. 36: 140. 1918, not Gagnepain (1917); Nipponorchis Masamune, nom. illeg. superfl. Herbs, epiphytic, small, monopodial. Roots many, slightly flattened. Stems erect, enclosed in leaf sheaths, short, many leaved. Leaves distichous, narrow, ± conduplicate toward base, dorsally carinate, jointed and sheathing at base. Inflorescence axillary, race- mose, laxly few flowered. Flowers opening widely, medium-sized. Sepals and petals free, similar; lip spurred at base, 3-lobed; lateral lobes erect; mid-lobe spreading, with appendages at base; spur slender, cylindric, rather long, sometimes slightly curved. Column short, thick, winged, footless; rostellum furcate-bifid; anther cap narrowed at apex; pollinia 2, cleft, waxy, globose; stipe narrowly ovate-cuneate, geniculate-curved; viscidium broadly ovate, broader than stipe. Three species: China, Japan, Korea; three species (two endemic) in China. 1a. Spur 3.5–5 cm ................................................................................................................................................................... 1. N. falcata 1b. Spur 1–1.6 cm. 2a. Sepals and petals pinkish or becoming whitish; spur 1.5–1.6 cm, much longer than sepals, curved, spreading horizontally, lateral sepals mucronate at apex ................................................................................. 2. N. xichangensis 2b. Sepals and petals white; spur 1–1.1 cm, shorter than or ca. as long as sepals, straight, pendulous or nearly so, lateral sepals not mucronate at apex ................................................................................................ 3. N. richardsiana 1. Neofinetia falcata (Thunberg) Hu, Rhodora 27: 107. 1925. obliquely bilobed. Inflorescence including flowers 4–5 cm, (2 or)3- or 4-flowered; peduncle 3–5 mm; floral bracts subovate, 风兰 feng lan 3–4 mm, scarious. -
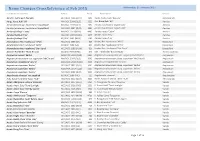
Plant Name Changes in Award Records X-Ref. Listing at 2015
Name Changes CrossReference at Feb 2015 Wednesday, 25 February 2015 Old Name as awarded Award AOC# Plant Name Genus Aerctm. Fuchs Gem 'Nerada' HCC/AOC 1996 (QLD) 1994 Aerdv. Fuchs Gem 'Nerada' Aeridovanda Aergs. Korat Koki 'Jill' AM/AOC 2004 (QLD) 3233 Aer. Korat Koki 'Jill' Aerides Aerides falcata var. houlletiana 'Liquid Gold' AM/AOC 1997 (QLD) 1990 Aerides houlletiana 'Liquid Gold' Aerides Aerides falcata var. houlletiana 'Liquid Gold' ACC/AOC 1997 (QLD) 1991 Aerides houlletiana 'Liquid Gold' Aerides Aerides fieldingii 'Cathy' AM/AOC 2011(NSW) 4485 Aerides rosea 'Cathy' Aerides Aerides fieldingii 'Fay' AM/AOC 1992 (NSW) 1245 Aerides rosea 'Fay' Aerides Aerides fieldingii 'Fay' AD/AOC 1992 (NSW) 1246 Aerides rosea 'Fay' Aerides Alexanderara Hec Hazelwood ‘APOC’ HCC/AOC 1989 (SA) 806 Brsdm. Hec Hazelwood 'APOC' Brassidium Alexanderara Hec Hazelwood ‘APOC’ AD/AOC 1989 (SA) 807 Brsdm. Hec Hazelwood 'APOC' Brassidium Alexanderara Hec Hazelwood ‘Star Burst’ HCC/AOC 1988 (NSW) 826 Brsdm. Hec Hazelwood 'Star Burst' Brassidium Aliceara Pathfinder ‘Black Beauty’ AD/AOC1989 (NSW) 824 Gbt. Pathfinder 'Black Beauty' Gombrassiltonia Angraecum bosseii 'Keiths' CBM/AOC 2001 (QLD) 2831 Angraecum sesquipedale var. angustifolium 'Keiths' Angraecum Angraecum eburneum var. superbum 'McClintock' AM/AOC 2003 (QLD) 3092 Angraecum eburneum subsp. superbum 'McClintock' Angraecum Angraecum magdalene 'Jorunn' ACM/AOC 2013 (NSW) 4938 Angraecum magdalenae 'Jorunn' Angraecum Angraecum superbum 'Keiths' HCC/AOC 1997 (QLD) 2091 Angraecum eburneum subsp. superbum 'Keiths' Angraecum Angraecum superbum 'Keiths' ACM/AOC 2014 (QLD) 5025 Angraecum eburneum subsp. superbum 'Keiths' Angraecum Angraecum superbum 'Keiths' ACC/AOC 2002 (QLD) 3013 Angraecum eburneum subsp. superbum 'Keiths' Angraecum Angulocaste clowessi ‘not supplied’ AD/AOC 1980 (VIC) 311 Angulocaste clowessi Angulocaste Ards. -

SOUTHERN ONTARIO ORCHID SOCIETY September 2018, Volume 53, Issue 8 Meeting Since 1965
SOUTHERN ONTARIO ORCHID SOCIETY September 2018, Volume 53, Issue 8 Meeting since 1965 Next Meeting Sunday, September 2, 2018, Floral Hall of the Toronto Botanical Garden. Vendor sales noon to 1pm 12:15 pm Cultural Snapshots by Alexi Antanaitis on the stage. Program at 1pm Fred Clarke of Sunset Valley Orchids in California. Fred will speak on his specialty Catasetum breeding. Member plant table. Bring in your flowering plants for show and tell and points Raffle President’s Remarks Welcome September 2, Fred Clarke, Sunset Valley Orchid Enthusiasts, The summer has flown by for Orchids (California), me, and the fact that it is now dark when I get up for (http://sunsetvalleyorchids.com) Catasetums work at 5:30am, is a reminder that the days are October 7, Jason Fischer, Orchids rapidly getting shorter. It is still quite hot (mid- Limited (Minnesota) August), and my orchids have been outside enjoying (https://www.orchidweb.com) the warmth, high humidity and the drenching rains November 4th: John Marcotte, Orchids that they have experienced lately. Soon the evenings Canada, (https://www.orchidscanada.com/), will start to get cooler, which for plants like Disas Phalaenopsis, will start setting their flower spikes. My Cattleyas are putting forth new growths that will December 2nd: Annual auction and pot luck flower this Fall and will result in peak blooms in December and January, if last year was any Our cultural snapshots will continue to take place on indication. the stage at 12:15 pm, starting in September. Alexsi Antanaitis will be running these sessions. Everyone is I hope that most of you were able to attend Orchidfest on July 8, with Robert Fuchs of RF Orchids in welcome to participate. -

Bulletin May-June 2021
Volume 36 Number 3 Bulletin May-June 2021 Committee Members Society meetings President: Mike Pieloor 0438 071 492 Meetings are now held on the first Wednesday of each Vice-President: Andrea Robold 0418 241 694 month (except January) at the Ainslie Football Club, Treasurer: Jane Wright 0406 379 054 52 Wakefield Ave, Ainslie, at 7:00 pm for a 7:30 pm start. Secretary: Bill Ferris 02 6297 5635 Visitors are welcome. Committee: Jenny Cooke 0419 497 078 Elisa Pavlic 0459 125 907 Karen Groeneveld 0418 414 632 Other Roles Next Meeting Public Officer Peter Coyne Wednesday 5 May 2021 at the Ainslie Football Club. Bulletin Editor Karen Groeneveld Mark Clements to talk about the genus Web Master Bill Ferris Bulbophyllum. Details on page 4. Conservation Officer Derek Corrigan Scientific Advisor Mark Clements Note the new starting time: Photography & Art Zoe Groeneveld * 7:00 pm for a 7:30 pm start * New Member Coordinator Jacquie Bannerman Meetings Entry Table Yvonne Day & Door Prizes Audrey Rough Popular Vote Jacquie Bannerman Upcoming Events Coordinators Jacqui Turner Benching Officer Mark Fraser 14-16 May: Orchids out West CANCELLED Judges Choice Robyn Noel Hawkesbury Race Club, Clarendon, NSW Krysia Szkiela Jane Wright 2 June (Wed): Society Meeting Librarian Andrea Robold Ainslie Football Club, 7:00pm for a 7:30pm start. Social Media Mike Pieloor AGM followed by Ben Wallace talking about CAM in Karen Groeneveld Mark Fraser orchids. Details on page 4. Sales Table Jane Wright 22-23 June 2021: Orchid Conservation Symposium Brenda Thompson A free, online event. Details on page 5. Bill Ferris Members also help in other ways, like storing 26-27 June: Mingara Orchid Club Fair & Show Society equipment or lending us resources; Mingara Recreation Club, Mingara Drive, Tumbi organising supper for meetings, and setting out and Umbi, NSW, Australia. -

SOOS October 2018
SOUTHERN ONTARIO ORCHID SOCIETY October 2018, Volume 53, Issue 9 Meeting since 1965 Next Meeting Sunday, October 7, 2018, Floral Hall of the Toronto Botanical Garden. Vendor sales noon to 1pm 12:15 pm Cultural Snapshots by Alexi Antanaitis on the stage. Program at 1pm Jason Fischer of Orchids Limited in Minnesota. Jason will give a talk on alternative growing methods which should be of interest to everyone. Member plant table. Bring in your flowering plants for show and tell and points Raffle Paphiopedilum Macabre Pops Vandachostylis Viboon Velvet” Cobalt Treasure” AM 80 grown ”CAD's Burgundy Wopper” AM 83 grown by David Bryan by Terry and Doug Kennedy President’s Remarks: Welcome Orchid By the time that you receive this newsletter, Laura Enthusiasts. Fall is around the corner, although it doesn’t feel like it as I write this mid-month, as we were Liebgott will have travelled to Cambridge to put on our experiencing height of summer temperatures. An early Society’s display for the Central Ontario Orchid Society September photo, sent from my daughter in Calgary Show on Sep.22-23.There are still more shows this fall, with snow on the cars, was a gentle reminder that cooler and Laura and Don Wyatt will once again be temperatures are coming. If your plants are still outdoors representing SOOS with beautiful award winning like mine are, then they are loving the warm weather and displays. They will be on the lookout for plants, so high humidity. I’ve been doing a lot of repotting lately, please help them out by sending some of your “babies” and I’m seeing great root growth. -

Plant-Pollinator Interactions in East Asia: a Review
Journal of Pollination Ecology, 25(6), 2019, pp 46-68 — Review — PLANT-POLLINATOR INTERACTIONS IN EAST ASIA: A REVIEW Daichi Funamoto*1,2 1Present address: Graduate School of Agricultural Science, Kobe University. Rokkodaicho 1-1, Nada, Kobe 657-8501, Japan 2School of Life Sciences, University of KwaZulu-Natal, P Bag X01, Scottsville, Pietermaritzburg 3209, South Africa Abstract—Pollination studies in East Asia have been developing rapidly in recent decades. East Asia may provide important information on many aspects of plant-pollinator interactions because of the rich fauna and flora and highly heterogeneous environments that occur there. In this review, plant-pollinator interactions in East Asia were summarized. Bumblebees are important pollinators of many plant species in East Asia, as well as in Europe and North America. Native honeybees may also have important roles in pollination in East Asia. Bird pollination and hawkmoth pollination may be less common in East Asia than in North America. Geographic variation in pollination interactions is expected because several types of pollinators are rare or absent in some habitats or geographic regions. For example, specialized nectar-feeding vertebrates like sunbirds and pteropodid bats are absent from most of East Asia except for some areas in its southern part. Opportunistic nectar-feeding vertebrates may have important roles in pollination where specialized nectar-feeding vertebrates are absent. Human impacts on plant pollinator interactions are understudied in this region. However, climate change, habitat degradation, and invasive species may have negative impacts on plant- pollinator interactions and thus plant reproductive success there. The information available on the plant-pollinator interactions in East Asia is still limited because many plant and pollinator taxa and many types of habitats are understudied. -

Potential Pollinator of Vanda Falcata (Orchidaceae): Theretra (Lepidoptera: Sphingidae) Hawkmoths Are Visitors of Long Spurred Orchid
NOTE Eur. J. Entomol. 112(2): 393–397, 2015 doi: 10.14411/eje.2015.031 ISSN 1210-5759 (print), 1802-8829 (online) Potential pollinator of Vanda falcata (Orchidaceae): Theretra (Lepidoptera: Sphingidae) hawkmoths are visitors of long spurred orchid KENJI SUETSUGU 1, KOJI TANAKA2, YUDAI OKUYAMA3 and TOMOHISA YUKAWA3 1 Graduate School of Human and Environmental Studies, Kyoto University, Yoshida-Nihonmatsu-cho, Sakyo, Kyoto 606-8501, Japan; e-mail: [email protected] 2 349 Yamamoto, Minami, Kokura, Kita-Kyushu, Fukuoka 803-0264, Japan; e-mail: [email protected] 3 Tsukuba Botanical Garden, National Museum of Nature and Science, Tsukuba, Ibaraki, Japan; e-mails: [email protected]; [email protected] Key words. Lepidoptera, Sphingidae, Theretra, Orchidaceae, Aeridinae, Vanda falcata, hawkmoth, orchid, pollinator Abstract. Vanda falcata is a species of orchid native to China, Korea and Japan. While it is arguably one of the most celebrated orchids in Japan there is no information on its pollinators. Although most species of the subtribe Aeridinae, to which V. falcata belongs, have a short spur, V. falcata has a long spur. The results of the current study provide strong evidence that V. falcata is pollinated by long- tongued hawkmoths (Theretra spp.), which indicates that the evolution of long spurs in V. falcata could be an adaptation to pollination by long-tongued moths. INTRODUCTION Vanda is comprised of approximately 70 epiphytic or lithophytic species and is widely distributed throughout East Asia in China, The unique morphology of orchid flowers has always been a Korea and Japan, and in Southeast Asia, from India and Nepal source of fascination and their floral diversity has inspired many southwards through the Philippines and Indonesia, all the way biologists since the 19th Century, including Charles Darwin (Dar- to northern Australia and the Solomon Islands (Govaerts, 2012; win, 1862).