Paradoxic Activation of the Renin-Angiotensin System in Twin–Twin Transfusion Syndrome: an Explanation for Cardiovascular Disturbances in the Recipient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

L&D – Amnioinfusion Guideline and Procedure for Amnioinfusion
L&D – Amnioinfusion Guideline and Procedure for Amnioinfusion. Purpose: Replacing the amniotic fluid with normal saline has been found to be a safe, simple, and very effective way to reduce the occurrence of repetitive variable decelerations. Procedure: Initiation of Amnioinfusion will be ordered and performed by a Certified Nurse Midwife (CNM) or physician (MD). 1. Prepare NS or LR 1000ml with IV tubing in the same fashion as for intravenous infusion. Flush the tubing to clear air. 2. An intrauterine pressure catheter (IUPC) will be placed by the MD/CNM. 3. Elevate the IV bag 3-4 feet above the IUPC tip for rapid infusion. Infuse 250-500ml of solution over a 20-30 minute time frame followed by a 60-180ml/hour maintenance infusion. The total volume infused should not exceed 1000ml unless one has access to ultrasound and can titrate to an amniotic fluid index (AFI) of 8-12 cm to prevent polyhydramnios and hypertonus. 4. If variable decelerations recur or other new non-reassuring FHR patterns develop, notify the MD/CNM. The procedure may be repeated as ordered. 5. Resting tone of the uterus will be increased during infusion but should not increase > 15mmHg from previous baseline. If this occurs, infusion should stop until there is a return to the previous baseline then it can be restarted. An elevated baseline prior to infusion is a contraindication. 6. Monitor for an outflow of infusion. If there is a sudden cessation of outflow fetal head engagement may have occurred increasing the risk of polyhydramnios. Complications are rare but can include iatrogenic polyhydramnios, uterine hypertonus, chorioamnionitis, uterine rupture, placental abruption, and maternal pulmonary embolus. -

Complicating Conditions Associated with Childbirth, by Delivery Method and Payer, 2011
HEALTHCARE COST AND Agency for Healthcare UTILIZATION PROJECT Research and Quality STATISTICAL BRIEF #173 May 2014 Highlights Complicating Conditions Associated With ■ Among the 3.6 million hospital Childbirth, by Delivery Method and Payer, stays involving childbirth in 2011 2011, cesarean section deliveries were 11 percent more Jennifer E. Moore, Ph.D., R.N., Whitney P. Witt, Ph.D., M.P.H., and likely among women who were Anne Elixhauser, Ph.D. covered by private insurance than among women covered by Introduction Medicaid. Mean length of stay and mean hospital costs were Childbirth is the most prevalent reason for hospitalization in the similar by payer type. United States.1,2 Of the 4.1 million hospital stays in 2009 involving childbirth, 91.3 percent of vaginal and 99.9 percent of ■ Among women who delivered cesarean section deliveries had at least one complicating by cesarean section and were condition.3 These conditions range in severity and may include covered by Medicaid, 94.6 those that are preexisting, such as mental health disorders; those percent of discharges included a that create risk factors, such as multiple gestation; and those that complicating condition. may lead to complications of care, such as an abnormality of fetal heart rate or rhythm. ■ Overall, for discharges among women with vaginal deliveries In the United States, childbirth accounts for about 10 percent of all covered by private insurance, maternal hospital stays and $12.4 billion in hospitalization costs the rate of cases with for live births; it represents, in the aggregate, one of the most complications increased with costly conditions for inpatient hospital care.4,5 The average cost age (75.5 per 100 for of a vaginal birth in 2008 was $2,900 without complications and adolescents younger than 15 $3,800 with complications.2 The average cost of a cesarean years versus 83.3 per 100 for section was $4,700 without complications and $6,500 with women aged 40–44 years). -

Fetal Cardiac Interventions—Are They Safe for the Mothers?
Journal of Clinical Medicine Article Fetal Cardiac Interventions—Are They Safe for the Mothers? Beata Rebizant 1,* , Adam Kole´snik 2,3,4, Agnieszka Grzyb 2,5 , Katarzyna Chaberek 1, Agnieszka S˛ekowska 1,6, Jacek Witwicki 7, Joanna Szymkiewicz-Dangel 2 and Marzena D˛ebska 1,8,* 1 2nd Department of Obstetrics and Gynecology, Centre of Postgraduate Medical Education, 01-809 Warsaw, Poland; [email protected] (K.C.); [email protected] (A.S.) 2 Department of Perinatal Cardiology and Congenital Anomalies, Centre of Postgraduate Medical Education, US Clinic Agatowa, 03-680 Warsaw, Poland; [email protected] (A.K.); [email protected] (A.G.); [email protected] (J.S.-D.) 3 Cardiovascular Interventions Laboratory, The Children’s Memorial Health Institute, 04-730 Warsaw, Poland 4 Department of Descriptive and Clinical Anatomy, Medical University of Warsaw, 02-004 Warsaw, Poland 5 Department of Cardiology, The Children’s Memorial Health Institute, 04-730 Warsaw, Poland 6 Pain Clinic, Department of Anesthesiology and Intensive Care, Centre of Postgraduate Medical Education, 00-416 Warsaw, Poland 7 Department of Neonatology, Centre of Postgraduate Medical Education, 01-809 Warsaw, Poland; [email protected] 8 Department of Gynecologic Oncology and Obstetrics, Centre of Postgraduate Medical Education, 00-416 Warsaw, Poland * Correspondence: Correspondence: [email protected] (B.R.); [email protected] (M.D.); Tel.: +48-508130737 (B.R.); +48-607449302 (M.D.) Abstract: The aim of fetal cardiac interventions (FCI), as other prenatal therapeutic procedures, is to bring benefit to the fetus. However, the safety of the mother is of utmost importance. -

A Pregnancy with GDM, Hypothyroidism, Polyhydramnios with History of Treatment for Subfertility
International Journal of Reproduction, Contraception, Obstetrics and Gynecology Mukherjee S et al. Int J Reprod Contracept Obstet Gynecol. 2018 May;7(5):2034-2037 www.ijrcog.org pISSN 2320-1770 | eISSN 2320-1789 DOI: http://dx.doi.org/10.18203/2320-1770.ijrcog20181951 Case Report A pregnancy with GDM, hypothyroidism, polyhydramnios with history of treatment for subfertility Soumya Mukherjee1, Sukanta Sen2*, Sukanta Misra3 1Department of Obstetrics and Gynecology, Nehru Memorial Techno Global Hospital, Barrackpur Cantonment, West Bengal, India 2Department of Pharmacology, ICARE Institute of Medical Sciences and Research, Haldia, West Bengal, India 3Department of Obstetrics and Gynecology, Ramakrishna Mission Seva Pratishthan Vivekananda Institute of Medical Sciences, Kolkata, West Bengal, India Received: 19 January 2018 Accepted: 28 February 2018 *Correspondence: Dr. Sukanta Sen, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Gestational diabetes (GDM) is defined as carbohydrate intolerance that begins or is first recognized during pregnancy. Although it is a well-known cause of pregnancy complications, its epidemiology has not been studied systematically. There are several identifiable predisposing factors for GDM, and in the absence of risk factors, the incidence of GDM is low. Low thyroid hormone levels in early pregnancy are a risk factor for GDM incidence. Although gestational hyperthyroidism is uncommon (0.2%), hypothyroidism (autoimmune disease or suboptimal iodine intake) occurs in 2.5% of women and is predictive of reduced neonatal and child neuropsychological development and maternal obstetric complications. -

Polyhydramnios: Causes, Diagnosis and Therapy Das Polyhydramnion: Ursachen, Diagnostik Und Therapie
Review 1241 Polyhydramnios: Causes, Diagnosis and Therapy Das Polyhydramnion: Ursachen, Diagnostik und Therapie Authors A. Hamza1, D. Herr 1, E. F. Solomayer2, G. Meyberg-Solomayer1 Affiliations 1 Gynäkologie und Geburtshilfe, Universitätsklinikum des Saarlandes, Homburg/Saar 2 Klinik für Frauenheilkunde, Geburtshilfe und Reproduktionsmedizin, Universitätsklinikum des Saarlandes, Homburg/Saar Key words Abstract Zusammenfassung l" polyhydramnios ! ! l" amniotic fluid Polyhydramnios is defined as a pathological in- Als Polyhydramnion bezeichnet man eine patho- l" high risk pregnancy crease of amniotic fluid volume in pregnancy logische Vermehrung von Fruchtwasser bei der Schlüsselwörter and is associated with increased perinatal mor- Schwangeren, die mit einer erhöhten perinatalen l" Polyhydramnion bidity and mortality. Common causes of Morbidität und Mortalität vergesellschaftet ist. l" Fruchtwasser polyhydramnios include gestational diabetes, fe- Häufige Ursachen eines Polyhydramnions sind l" Risikoschwangerschaft tal anomalies with disturbed fetal swallowing of der Gestationsdiabetes, fetale Fehlbildungen, die amniotic fluid, fetal infections and other, rarer z.B. zu einem gestörten Schluckvorgang von causes. The diagnosis is obtained by ultrasound. Fruchtwasser führen, fetale Infektionen und an- The prognosis of polyhydramnios depends on its dere seltene Ursachen. Die Diagnostik des Poly- cause and severity. Typical symptoms of hydramnions erfolgt dabei v.a. sonografisch. Die Deutschsprachige polyhydramnios include maternal dyspnea, -

Chapter 14 CORD PROLAPSE
Perinatal Manual of Southwestern Ontario Southwestern Ontario Maternal, Newborn, Child & Youth Network (MNCYN) Perinatal Outreach Program Chapter 14 CORD PROLAPSE Cord prolapse occurs in 1/200 to 1/400 pregnancies. Perinatal mortality ranges from 0.02% to 12.6%. The outlook for the fetus is influenced by the degree and duration of cord compression and the time interval between the diagnosis and birth. Definition: Umbilical cord prolapse is defined as the descent of the umbilical cord through the cervix alongside (occult) or past the presenting part (overt) in the presence of ruptured membranes. Cord presentation is the presence of the umbilical cord between the fetal presenting part and the cervix, with or without membrane rupture Risk Factors 1. Malpresentation: more common when preterm, multiple gestation, polyhydramnios, pelvic tumors 2. Unstable lie 3. Hydramnios 4. Grand multiparous women i.e., parity of >5 5. Rupture of membranes when the presenting part is high 6. Preterm rupture of membranes 7. CPD 8. Placenta previa, low lying placenta 9. Male gender 10. Fetal congenital anomalies 11. Birth weight less than 2500 g 12. Pelvic tumours Presentation 1. Sudden fetal bradycardia 2. Patient complaint that something is coming from the vagina 3. Visual – umbilical cord seen at introitus (majority are hidden in vagina, therefore, diagnosis is not always easy) 4. Palpation of the cord on vaginal examination Revised October 2018 14-1 Disclaimer The Southwestern Ontario Maternal, Newborn, Child & Youth Network (MNCYN) has used practical experience and relevant legislation to develop this manual chapter. We recommend that this chapter only be used as a reference document at other facilities. -

Evaluation of the Placenta and Cervix
Evaluation of the Placenta Disclaimer and Cervix • I have no relevant financial relationships Judy A. Estroff, MD with the manufacturer(s) of any commercial product(s) and/or provider(s) of any commercial services discussed in this CME activity. • I do not intend to discuss unapproved or Boston Children’s Hospital investigative use of a commercial Harvard Medical School product/device in my presentation. Boston, MA Overview Everything you need to know in • Amniotic fluid 15 minutes! • Placenta • Umblical cord • Cervix • Membranes Amniotic fluid volume Amniotic Fluid • Increases logarithmically first ½ pregnancy • Definitions • < 10 mL @ 8 weeks gestation • Classification • 630 mL @ 22 weeks gestation • 770 mL @ 28 weeks gestation • 30-36 weeks: volume stable or slowly inc • > 36 weeks: volume decreases • 41 weeks: 515 mL • Decreases 33% each week after 41 weeks Creasy & Resnik: Maternal Fetal Medicine 6th Edition, 2009 1 Measurement of amniotic fluid • AFI= Amniotic fluid index • Subjective assessment • Deepest vertical pocket AFI: Amniotic Fluid Index x • Definition: Summation of the deepest vertical pocket (DVP) in 4 cord and extremity- free quadrants of the gravid uterus • Oligohydramnios: < 5 cm • Polyhydramnios: > 24 cm 27w DVP=13.3 cm Oligohydramnios Oligohydramnios • Definition: Condition in which the amniotic fluid volume (AFV) is • Almost always associated with an decreased relative to gestational age. increased risk of fetal morbidity and mortality • Or: AFI < 300-500 mL in 2nd trimester • MVP < 1-2 cm •AFI < 5 cm • AFI < 5% -

Section I: Scenario Overview
SECTION I: SCENARIO OVERVIEW Scenario Title: Prolapsed Umbilical Cord MCH Original Scenario Developer(s) C. Lopez, RNC, MSN, CNS, S. Cantrell, MSN, RNC Date - original scenario 04/08 Validation: 02/09 Revision Dates: 08/18 Connie Lopez, MSN, CNS, RNC-OB, CPHRM, CHSEA 08/10 Pilot testing: 02/09 QSEN revision: Estimated Scenario Time: 15 minutes Debriefing time: 30 minutes Target group: Pre-licensure students in OB, new graduates, orientees, staff in OB and IP teams Core case: Prolapsed Umbilical Cord/Emergency C-section QSEN/IOM Competencies: Patient Centered Care Safety Teamwork and Collaboration Brief Summary of Case: Carey Jones is a 22-year-old woman who is G3P0 at 37 weeks gestation. She was admitted to L&D 3 hours ago, in early labor. She had a moderate variable deceleration (< 70 bpm lasting 60 seconds) during her triage evaluation. FHR has had moderate variability with accelerations, since that time. Early prenatal care was uneventful. However, at 33 weeks, it was noted that fetal size was greater than dates and an ultrasound confirmed fetal growth and anatomy were within normal limits. Her AFI was 29 and a diagnosis of polyhydramnios was confirmed. She is 5'5" and weighs 220 lbs. Her blood sugars have been within normal limits. EVIDENCE BASE / REFERENCES (APA Format) Davidson, M. R., London, M. L., & Ladewig, P. W. (2012). Olds maternal-newborn nursing & womens health across the lifespan. Boston: Pearson. Philadelphia, Saunders. Gaumard Scientific, Miami, FL. (2018) Noelle/Victoria maternal and neonatal simulation system: Instructor guide. Retrieved from https://www.gaumard.com/dowithin normal limitsoads Laredal Medical Corporation, Wappingers Falls, NY. -

Breech Presentation Guidelines
Breech Presentation External Cephalic Version and Vaginal Breech Delivery Document Control Title Breech Presentation, External Cephalic Version and Vaginal Breech Delivery Guideline Author Author’s job title Obstetric Consultant, LW Lead Obstetric Specialty Doctor Lead Clinical Midwife Directorate Department Womens and Childrens Maternity Services Date Version Status Comment / Changes / Approval Issued 1.0 Sep 2002 Final Original version 1.1 Jun 2012 Revision Updated 1.2 Feb 2013 Revision Revision 1.3 May 2013 Revision Guideline converted to Trust template. 2.0 June Final Revision 2013 3.0 July 2017 Final Revision 3.1 Sept Final Approved for publishing 2020 Main Contact Consultant Obstetrician, Labour Ward Tel: Direct Dial – Lead Tel: Internal – North Devon District Hospital Email: Raleigh Park, Barnstaple, EX31 4JB Lead Director Director of Nursing Superseded Documents Issue Date Review Date Review Cycle September 2020 September2023 Three years Consulted with the following stakeholders: (list all) Obstetricians Midwives Guidelines group stakeholders All users of this document Approval and Review Process Maternity Services Guidelines Group Local Archive Reference Maternity Services Risk Manager Local Path G:\OBSGYNAE\Risk\Archives\Maternity Services Filename Breech Presentation External Cephalic Version and Vaginal Breech Delivery v3.0 May2017 Policy categories for Trust’s internal Tags for Trust’s internal website (Bob) website (Bob) Maternity Services Malpresentation vaginal birth Obs&Gynae Page 1 of 20 Breech Presentation External -

Evaluation of Pregnancy Outcomes in Gestational Diabetes Mellitus
Orginal Article Medical Journal of Pokhara Academy of Health Sciences (MJPAHS) Vol. 1 Issue 2 Jul-Dec 2018 Evaluation of Pregnancy Outcomes in Gestational Diabetes Mellitus Lakshmi Sunar1, Zhu Yan2 1Department of Obstetrics and Gynaecology, Western Regional Hospital, Pokhara Academy of Health Sciences, Pokhara, Gandaki, Nepal 2Department of Obstetrics and Gynaecology, First Affiliated Hospital of Liaoning Medical University,Jinzhou, Liaoning, P.R.China ABSTRACT Corresponding author: Objectives: To evaluate the pregnancy outcomes in the Dr. Lakshmi Sunar, MD patients diagnosed with Gestational Diabetes Mellitus. Obstetrician & Gynaecologist Materials and Methods: A retrospective study conducted Department of Obstetrics and Gynaecology on ninety-two patients, delivered in the First Affiliated Hospital Pokhara Academy of Health Sciences of Liaoning Medical University, China from February 2014 to Western Regional Hospital June 2015. [email protected] Results: The rate of Cesarean section was 36.95%, polyhydramnios 27.17%, macrosomia 21.73% and preterm delivery was14.13% respectively. Article recived : 17th Jan. 2018 Conclusion: Gestational Diabetes Mellitus is recognized Article accepted : 4thOct. 2018 to be associated with increased rate of adverse pregnancy outcomes. This study demonstrated that the GDM has higher risk for polyhydramnios and macrosomia. Key words : Blood glucose, Gestational Diabetes Mellitus, Pregnancy outcome. INTRODUCTION MATERIALS AND METHODS Gestational Diabetes Mellitus (GDM) is defined as A retrospective study conducted on ninety-two “onset or first recognition of any degree of glucose patients, delivered in the First Affiliated Hospital of 1 intolerance of variable severity during pregnancy”. Liaoning Medical University, China from February Recently in 2012, American Diabetes Association 2014 to June 2015. Selected patients were Chinese (ADA) described GDM as “diabetes diagnosed during Asian with the mean age of 28.98±3.23. -

Understanding Factors Leading to Primary Cesarean Section
www.nature.com/scientificreports OPEN Publisher Correction: Understanding Factors Leading to Primary Cesarean Section and Vaginal Birth After Cesarean Delivery in the Friuli‑Venezia Giulia Region (North‑Eastern Italy), 2005–2015 L. Cegolon, G. Mastrangelo, G. Maso, G. Dal Pozzo, L. Ronfani, A. Cegolon, W. C. Heymann & F. Barbone Correction to: Scientifc Reports https://doi.org/10.1038/s4159 8-019-57037 -y , published online 15 January 2020 Te original version of this Article contained several typographical errors. In the Abstract, “In our study we examined patterns of PCS, pl compared with planned TOLAC anned PCS (PPCS), vaginal birth afer 1 previous CS (VBAC-1) and associated factors in Friuli Venezia Giulia (FVG), a region of North-Eastern Italy, collecting data from its 11 maternity centres (coded from A to K) during 2005–2015.” now reads: “In our study we examined patterns of PCS, planned PCS (PPCS), vaginal birth afer 1 previous CS (VBAC-1) and associated factors in Friuli Venezia Giulia (FVG), a region of North-Eastern Italy, collecting data from its 11 maternity centres (coded from A to K) during 2005–2015.” In the Methods section under the subheading ‘Ethics Statement’, “According to the Italian privacy law (Legislative Decree 101/2018, D.Lgs 101/2018) regional NHS data can be used for scientifc purposes within the frame of approved studies/protocols.” now reads: “According to the Italian privacy law (Legislative Decree 101/2018, D.Lgs 101/2018) regional data from the Ital- ian National Health Service (NHS) can be used for scientifc -
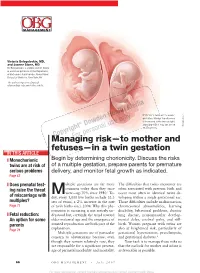
Managing Risk—To Mother and Fetuses—In a Twin Gestation
Victoria Belogolovkin, MD, and Joanne Stone, MD Dr. Belogolovkin is a fellow and Dr. Stone is associate professor in the Department of Maternal–Fetal Medicine, Mount Sinai School of Medicine, New York, NY The authors report no fi nancial relationships relevant to this article. Dichorionic twins at 11+ weeks' gestation. Nuchal translucency is increased in the twin at right, LaRocco ® signaling that it may be at risk Rich Dowden Health Media © of aneuploidy. Copyright ManagingFor personal risk—to use only mother and fetuses—in a twin gestation IN THIS ARTICLE ❙ Monochorionic Begin by determining chorionicity. Discuss the risks twins are at risk of of a multiple gestation, prepare parents for premature serious problems delivery, and monitor fetal growth as indicated. Page 67 ❙ Does prenatal test- ultiple gestations are far more The diffi culties that twins encounter are ing raise the threat common today than they once often associated with preterm birth and Mwere—up 70% since 1980.1 To- occur most often in identical twins de- of miscarriage with day, every 1,000 live births include 32.3 veloping within a single gestational sac. multiples? sets of twins, a 2% increase in the rate Those diffi culties include malformation, Page 77 of twin births since 2004. Why this phe- chromosomal abnormalities, learning nomenon is occurring is not entirely un- disability, behavioral problems, chronic ❙ Fetal reduction: derstood but, certainly, the trend toward lung disease, neuromuscular develop- An option for some older maternal age and the emergence of mental delay, cerebral palsy, and still- parents assisted reproduction are both part of the birth.