Efficacy and Safety of Dotinurad in Hyperuricemic Patients with Or Without Gout: a Systematic Review and Meta-Analysis of Randomized Controlled Trials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Specifications of Approved Drug Compound Library
Annexure-I : Specifications of Approved drug compound library The compounds should be structurally diverse, medicinally active, and cell permeable Compounds should have rich documentation with structure, Target, Activity and IC50 should be known Compounds which are supplied should have been validated by NMR and HPLC to ensure high purity Each compound should be supplied as 10mM solution in DMSO and at least 100µl of each compound should be supplied. Compounds should be supplied in screw capped vial arranged as 96 well plate format. -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine -

Cross-Over Trial of Febuxostat and Topiroxostat for Hyperuricemia with Cardiovascular Disease (TROFEO Trial)
Circ J 2017; 81: 1707 – 1712 ORIGINAL ARTICLE doi: 10.1253/circj.CJ-17-0438 Preventive Medicine Cross-Over Trial of Febuxostat and Topiroxostat for Hyperuricemia With Cardiovascular Disease (TROFEO Trial) Akira Sezai, MD, PhD; Kazuaki Obata; Keisuke Abe; Sakie Kanno; Hisakuni Sekino, MD, PhD Background: We previously reported that febuxostat was more effective for hyperuricemia than allopurinol. The efficacy, however, of topiroxostat (a novel xanthine oxidase reductase inhibitor similar to febuxostat), for hyperuricemia is unknown. Methods and Results: Patients with cardiovascular disease and hyperuricemia, in whom serum uric acid (s-UA) was controlled at ≤6 mg/dL, were eligible for enrollment. Fifty-five patients were randomized to receive either febuxostat or topiroxostat for 6 months and were switched to the other drug for the following 6 months. The primary endpoint was s-UA. Secondary endpoints included serum creatinine, estimated glomerular filtration rate, urinary albumin, cystatin-C, oxidized low-density lipoprotein, eicosapentaenoic acid/ arachidonic acid ratio, lipid biomarkers, high-sensitivity C-reactive protein and B-type natriuretic protein. Although s-UA level was similar for both drugs, significantly more patients required dose escalation during treatment with topiroxostat. There were no differ- ences in renal function, inflammatory and lipid markers between the 2 drugs. A biomarker of oxidative stress was significantly lower after 3 months of febuxostat compared with topiroxostat. Conclusions: Febuxostat causes more marked and more rapid reduction of s-UA than topiroxostat. With regard to the antioxidant effect, febuxostat was superior to topiroxostat after 3 months. The renal protective and anti-inflammatory effects of both drugs were also similar after 6 months of treatment. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Report on the Deliberation Results May 8, 2013 Evaluation And
Report on the Deliberation Results May 8, 2013 Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau Ministry of Health, Labour and Welfare [Brand name] (a) Topiloric Tablets 20 mg, 40 mg, and 60 mg (b) Uriadec Tablets 20 mg, 40 mg, and 60 mg [Non-proprietary name] Topiroxostat (JAN*) [Applicant] (a) Fujiyakuhin Co., Ltd. (b) Sanwa Kagaku Kenkyusho Co., Ltd. [Date of application] June 26, 2012 [Results of deliberation] In the meeting held on April 26, 2013, the First Committee on New Drugs concluded that the product may be approved and that this result should be presented to the Pharmaceutical Affairs Department of the Pharmaceutical Affairs and Food Sanitation Council. The product is not classified as a biological product or a specified biological product, the re-examination period is 8 years, and neither the drug substance nor the drug product is classified as a poisonous drug or a powerful drug. *Japanese Accepted Name (modified INN) This English version of the Japanese review report is intended to be a reference material to provide convenience for users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. The PMDA will not be responsible for any consequence resulting from the use of this English version. Review Report April 15, 2013 Pharmaceuticals and Medical Devices Agency The results of a regulatory review conducted by the Pharmaceuticals and Medical Devices Agency on the following pharmaceutical product submitted for registration are as follows. [Brand name] (a) Topiloric Tablets 20 mg, 40 mg, and 60 mg (b) Uriadec Tablets 20 mg, 40 mg, and 60 mg [Non-proprietary name] Topiroxostat [Applicant] (a) Fujiyakuhin Co., Ltd. -

Metrochem API Private Limited
Metrochem API Private Limited APIs USDMF / CEP Numbers Submission Date Status Written Confirmation Acotiamide Alogliptin Benzoate Antazoline HCL CEP 2020-060 10 February,2020 Antazoline Phosphate USDMF 34330 1 January,2020 Apixaban USDMF 35518 30 December,2020 Apremilast Azilsartan Baloxavir Marboxil Product Under Validation Bempedoic Acid Bepotastine Besilate Bilastine Canagliflozin Cenobamate Cinitapride Clopidogrel Bisulfate Form I USDMF 34334 5 February,2020 Indian CDSCO WC-0493 Clopidogrel Bisulfate Form II USDMF 34334 5 February,2020 Indian CDSCO WC-0493 Crisaborole Dabigatran Dapaglifloin Amorphous Dapagliflozin Dexketoprofen Trometamol USDMF 34732 21 March,2020 Indian CDSCO WC-0493 Dexlansoprazole USDMF 34971 29 September,2020 Dexrabeprazole Dimethindene Maleate CEP 2021-080 6 February,2021 Edoxaban Efinaconazole Elagolix Elobixibat Product Under Validation Eltrombopag Empagliflozin USDMF 35345 31 October,2020 Indian CDSCO WC-0493 Empagliflozin Amorphous Ertugliflozin Esomeprazole Magnesium Dihydrate USDMF 35519 ; CEP 2021-022 29 December,2020 ; 28 December 2020 Note: None of the products will be supplied to the countries in which this could be in conflict with existing patents. Further, any products under patent will be offered for R&D purpose only. However, the final responsibility exclusively lies with the buyer. Metrochem API Private Limited APIs USDMF / CEP Numbers Submission Date Status Written Confirmation Esomeprazole Magnesium Trihydrate USDMF 35400 ; CEP 2020-386 28 November,2020 ; 21 November,2020 Esomeprazole Sodium -

Protective Effects of Topiroxostat on an Ischemia-Reperfusion Model of Rat Hearts
Circ J 2018; 82: 1101 – 1111 ORIGINAL ARTICLE doi: 10.1253/circj.CJ-17-1049 Ischemic Heart Disease Protective Effects of Topiroxostat on an Ischemia-Reperfusion Model of Rat Hearts Shogo Tanno, BSc; Kenshiro Yamamoto, BSc; Yasutaka Kurata, MD, PhD; Maya Adachi, BSc; Yumiko Inoue, BSc; Naoyuki Otani, MD, PhD; Mutsuo Mishima, BSc; Yasutaka Yamamoto, MD, PhD; Masanari Kuwabara, MD, PhD; Kazuhide Ogino, MD, PhD; Junichiro Miake, MD, PhD; Haruaki Ninomiya, MD, PhD; Yasuaki Shirayoshi, PhD; Futoshi Okada, PhD; Kazuhiro Yamamoto, MD, PhD; Ichiro Hisatome, MD, PhD Background: Ischemia/reperfusion (I/R) injury triggers cardiac dysfunctions via creating reactive oxygen species (ROS). Because xanthine oxidase (XO) is one of the major enzymes that generate ROS, inhibition of XO is expected to suppress ROS-induced I/R injury. However, it remains unclear whether XO inhibition really yields cardioprotection during I/R. The protective effects of the XO inhibitors, topiroxostat and allopurinol, on cardiac I/R injury were evaluated. Methods and Results: Using isolated rat hearts, ventricular functions, occurrence of arrhythmias, XO activities and thiobarbituric acid reactive substances (TBARS) productions and myocardial levels of adenine nucleotides before and after I/R, and cardiomyocyte death markers during reperfusion, were evaluated. Topiroxostat prevented left ventricular dysfunctions and facilitated recovery from arrhythmias during I/R. Allopurinol and the antioxidant, N-acetylcysteine (NAC), exhibited similar effects at higher concentrations. Topiroxostat inhibited myocardial XO activities and TBARS productions after I/R. I/R decreased myocardial levels of ATP, ADP and AMP, but increased that of xanthine. While topiroxostat, allopurinol or NAC did not change myocardial levels of ATP, ADP or AMP after I/R, all of the agents decreased the level of xanthine. -

“M/S. AJANTA PHARMA LTD.”
APPLICATION FOR ENVIRONMENTAL CLEARANCE OF PROPOSED EXPANSION OF ACTIVE PHARMACEUTICAL INGREDIENTS (API), MANUFACTURING UNIT “M/s. AJANTA PHARMA LTD.” 11KM Stone, Gut No. 378, Plot No 8, Aurangabad –Pune Highway, Village-Waluj, Taluka. Gangapur, District. Aurangabad- 431133. FORM 1 Submitted to Expert Appraisal Committee (Industry-2), MoEFCC, New Delhi Submitted by M/s. AJANTA PHARMA LTD. Environmental Consultant: Building Environment (India) Pvt. Ltd Dakshina Building, Office No. 401, Plot No. 2, Sector-11, CBD Belapur, Navi Mumbai- 400 614 January, 2018. Form 1 for proposed Expansion of API Manufacturing Industry “M/s. Ajanta Pharma Ltd.” at Waluj Village, Taluka-Gangapur, District- Aurangabad, Maharashtra. Form – 1 (I) Basic Information:- Sr. Items Details No. 1. Name of the project Proposed expansion of Active Pharmaceutical Ingredients (API) Manufacturing Industry “M/s. Ajanta Pharma Ltd.” 2. S. No. in the schedule Category 5f as per EIA Notification 2006 & amendments 3. Proposed capacity/ area/ length/ Industry is already engaged in manufacturing 85 nos. of tonnage to be handled/ command API and having production capacity 21.042 MT/Month. area/lease area/ number of wells to In the proposed expansion industry will manufacture 256 be drilled nos. API including existing API. The total production capacity after expansion would remain same as existing i.e. 21.042 MT/Month. Annexure-1 : Details of existing & proposed API 4. New / Expansion / Modernization Expansion. Annexure -2: EC letter of existing unit 5. Existing Capacity/ Area etc. Industry is already engaged in manufacturing 85 nos. of API and having production 21.042 MT/Month. Annexure-3 : Details of existing API 6. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -
Detailed Program and Abstracts
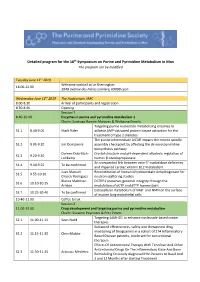
Detailed program for the 18th Symposium on Purine and Pyrimidine Metabolism in Man The program can be modified Tuesday June 11th 2019 Welcome cocktail at Le Sherrington 18.00-22.00 204B avenue des Frères Lumière, 69008 Lyon Wednesday June 12th 2019 The Auditorium, IARC 8.00-8.30 Arrival of participants and registration 8.30-8.40 Opening Session 1 8.40-10.40 Enzymes in purine and pyrimidine metabolism 1 Chairs: Santiago Ramón-Maiques & Wolfgang Knecht Targeting purine nucleotide metabolizing enzymes to S1.1 8.40-9.05 Mark Rider achieve AMP-activated protein kinase activation for the treatment of type 2 diabetes The purine intermediate AICAR impairs the mitotic spindle S1.2 9.05-9.20 Jim Dompierre assembly checkpoint by affecting the de novo pyrimidine biosynthesis pathway Doreen Dobritzch- Crystal structure and pH-dependent allosteric regulation of S1.3 9.20-9.40 Lohkamp human β-ureidopropionase An unexpected link between ecto-5’-nuclotidase deficiency S1.4 9.40-9.55 To be confirmed and impaired cardiac vitamin B12 metabolism Juan Manuel Reconstitution of human dihydroorotate dehydrogenase for S1.5 9.55-10.10 Orozco Rodriguez neutron scattering studies Blanca Martinez- DCTPP1 preserves genomic integrity through the S1.6 10.10-10.25 Arribas modulation of dCTP and dTTP homeostasis Extracellular metabolism of NAD+ and NMN on the surface S1.7 10.25-20.40 To be confirmed of murine lung endothelial cells 10.40-11.00 Coffee break Session 2 11.00-13.00 Drug development and targeting purine and pyrimidine metabolism Chairs: Suzanne Peyrottes & -

Jasvinder Singh, MD
Singh, Jasvinder MD, MPH CURRICULUM VITAE Date: April 30, 2020 PERSONAL INFORMATION: Name: Jasvinder A. Singh, MD, MPH Citizenship: U.S.A. RANK/TITLE: Departments: ! Endowed Professor, Musculoskeletal Outcomes Research Professor of Medicine and Epidemiology with Tenure Staff Physician, Birmingham VA Medical Center Business Address: ! University of Alabama at Birmingham Faculty Office Tower 805B 510 20th Street South Birmingham, AL 35294 HOSPITAL AND OTHER (NON ACADEMIC) APPOINTMENTS: 2009-present ! Staff Physician University of Alabama Hospital and University of Alabama Health Services Foundation 2009-present ! Staff Physician, Medical Service - Rheumatology Veterans Affairs (VA) Medical Center, Birmingham, Alabama 2009-present ! Staff Physician UAB Highlands Hospital, Birmingham, Alabama 2009-2012 ! Staff Physician Cooper Green Mercy Hospital, Birmingham, Alabama 2001- 2009 ! Staff Physician, Medical Service - Rheumatology Veterans Affairs (VA) Medical Center, Minneapolis, Minnesota EDUCATION: 2001-2003 ! Master of Public Health (Epidemiology) University of Minnesota, Minneapolis, MN 1988-1993 ! Bachelor of Medicine and Bachelor of Surgery (M.B.B.S.) University College of Medical Sciences, New Delhi, India POSTDOCTORAL TRAINING: 1998-2001 ! Rheumatology Fellowship, Division of Rheumatology and Immunology 1 Singh, Jasvinder MD, MPH Washington University School of Medicine, St. Louis, Missouri 1995-1998 ! Internship and Residency, Internal Medicine State University of New York (SUNY), Syracuse, New York 1994 Residency, Department of Psychiatry