Establishing a Baseline of Plant Diversity and Endemism on a Neotropical Mountain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primulaceae Flora of the Cangas of Serra Dos Carajás, Pará, Brazil: Primulaceae
Rodriguésia 68, n.3 (Especial): 1085-1090. 2017 http://rodriguesia.jbrj.gov.br DOI: 10.1590/2175-7860201768346 Flora das cangas da Serra dos Carajás, Pará, Brasil: Primulaceae Flora of the cangas of Serra dos Carajás, Pará, Brazil: Primulaceae Maria de Fátima Freitas1,2 & Bruna Nunes de Luna1 Resumo Este estudo apresenta as espécies de Primulaceae registradas para as áreas de canga da Serra dos Carajás, estado do Pará, incluindo descrição morfológica, comentários e ilustrações. São registrados dois gêneros e seis espécies para a área de estudo: Clavija lancifolia subsp. chermontiana, C. macrophylla, Cybianthus amplus, C. detergens, C. penduliflorus e Cybianthus sp. 1. Palavras-chave: Myrsinaceae, Theophrastaceae, FLONA Carajás, flora, taxonomia. Abstract This study presents the species of Primulaceae recorded for the cangas of Serra dos Carajás, Pará state, including morphological descriptions, comments and illustrations. Six species representing two genera were recorded in the study area: Clavija lancifolia subsp. chermontiana, C. macrophylla, Cybianthus amplus, C. detergens, C. penduliflorus e Cybianthus sp. 1. Key words: Myrsinaceae, Theophrastaceae, FLONA Carajás, flora, taxonomy. Primulaceae As espécies encontradas na cangas da Serra de Primulaceae apresenta distribuição Carajás caracterizam-se por serem arbusto a pantropical, com aproximadamente 2.500 arvoretas, bissexuais ou unissexuais, com folhas espécies e 58 gêneros (Stevens 2001 em diante) simples, alternas, não estipuladas. Apresentam agrupados em quatro subfamílias: Maesoideae, inflorescências racemosas, flores pediceladas, Myrsinoideae, Primuloideae e Theophrastoideae bractéola única, cálice livre ou fusionado na base (APG IV). Destas, duas ocorrem na Flora do e corola gamopétala. O androceu é tipicamente Brasil, Myrsinoideae e Theophrastoideae, com epipétalo, isostêmone, com estames livres entre 12 gêneros e cerca de 140 espécies (Freitas et si ou unidos em um tubo; estaminódios também al. -

Growing Alcantarea
Bromeliaceae VOLUME XLII - No. 3 - MAY/JUNE 2008 The Bromeliad Society of Queensland Inc. P. O. Box 565, Fortitude Valley Queensland, Australia 4006, Home Page www.bromsqueensland.com OFFICERS PRESIDENT Olive Trevor (07) 3351 1203 VICE PRESIDENT Anne McBurnie PAST PRESIDENT Bob Reilly (07) 3870 8029 SECRETARY Chris Coulthard TREASURER Glenn Bernoth (07) 4661 3 634 BROMELIACEAE EDITOR Ross Stenhouse SHOW ORGANISER Bob Cross COMMITTEE Greg Aizlewood, Bruce Dunstan, Barry Kable, Arnold James,Viv Duncan, David Rees MEMBERSHIP SECRETARY Roy Pugh (07) 3263 5057 SEED BANK CO-ORDINATOR Doug Parkinson (07) 5497 5220 AUDITOR Anna Harris Accounting Services SALES AREA CASHIER Norma Poole FIELD DAY CO-ORDINATOR Ruth Kimber & Bev Mulcahy LIBRARIAN Evelyn Rees ASSISTANT SHOW ORGANISER Phil Beard SUPPER STEWARDS Nev Ryan, Barry Genn PLANT SALES Pat Barlow Phil James COMPETITION STEWARDS Dorothy Cutcliffe, Arnold James CHIEF COMPETITION STEWARD HOSTESS Gwen Parkinson BSQ WEBMASTER Ross Stenhouse LIFE MEMBERS Grace Goode OAM Peter Paroz, Michael O’Dea Editors Email Address: [email protected] The Bromeliad Society of Queensland Inc. gives permission to all Bromeliad Societies to re- print articles in their journals provided proper acknowledgement is given to the original author and the Bromeliaceae, and no contrary direction is published in Bromeliaceae. This permission does not apply to any other person or organisation without the prior permission of the author. Opinions expressed in this publication are those of the individual contributor and may not neces- sarily reflect the opinions of the Bromeliad Society of Queensland or of the Editor Authors are responsible for the accuracy of the information in their articles. -
Ber Gl an D V on G U a Y A
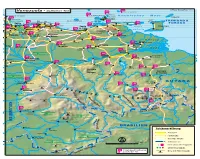
ISLAS LOS ROQUES ©REISE KNOW-HOW 2012 Venezuela - östlicher Teil 629 622 ISLAS LOS TESTIGOS 586 ISLAS LOS FRAILES San Juan de los Cayos ISLA MARGARITA Karibisches Meer 578 La Asunción TOBAGO Chichiriviche Porlamar Punta 268 PORT TRINIDAD & o Nationalpark 209 La Los ISLA LA TORTUGA de Piedras OF SPAIN Morrocoy GuairaCaracas Carúpano 289 Puerto Maiquetía 216 245 TOBAGO Morón Choroní Chuspa Irapa Güiria Cabello 169 Higuerote Cumaná Cariaco Casanay 558 537 Guatire Puerto Golfo TRINIDAD Henri Maracay CARACAS Caucagua Cueva del Valencia Pittier 226 La Cruz Cagua El Guapo Guácharo deParia San Fernando Nat. Park Cua Barcelona San Caripito a Bejuma Francisco Villa de 431 Cura Santa Fé La Encrucijada La Toscana 299 Tinaquillo San Juan Altagracia de los Morros Maturín Tinaco de Orituco Aragua de Barcelona 316 Anaco Orinoco- Chaguaramas La Horqueta El Sombrero Zaraza 306 Los Güires 436 Tucupita 434 Valle de El Tigrito Temblador El Baúl la Pascua 317 San Antonio Calabozo El Tigre de Tabasca Delta Moja 322 Barrancas Casabe Ciudad Nationalpark Guayana 333 Aguaro-Guariquito Río Gua Ciudad 361 Campamento na r 416 Río Grande e Bolívar Upata San Fernando Apurito Moitaco El Palmar de Apure Mapire Achaguas Cabruta 398 R Caicara 345 ío A Bochinche pure del Orinoco Las Ciudad ual o Adjuntas 363 c Piar Embalse 363 o n de Guri ri El Callao Tumeremo O El Manteco Río Serranía La Urbana Serranía Turagua 345 de Imataca La Vergareña Paragua 364 Nationalpark Guaniamo El Dorado Santos Luzardo 410 Puerto Río ío Meta C R a Páez u GUYANA Puerto r San Isidro a 346 Carreño -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Rediscovery of Cybianthus Froelichii (Primulaceae), an Endangered Species from Brazil
Bol. Mus. Biol. Mello Leitão (N. Sér.) 38(1):31-38. Janeiro-Março de 2016 31 Rediscovery of Cybianthus froelichii (Primulaceae), an endangered species from Brazil Maria de Fátima Freitas1*, Tatiana Tavares Carrijo2 & Bruna Nunes de Luna1 RESUMO: (Redescoberta de Cybianthus froelichii (Primulaceae), uma espécie ameaçada de extinção do Brasil). Uma espécie redescoberta de Cybianthus subgênero Cybianthus é descrita e ilustrada. Cybianthus froelichii é proximamente relacionado à C. cuneifolius, mas se diferencia pelas folhas grandes e flores pistiladas sésseis. Espécie endêmica da Mata Atlântica e considerada ameaçada de extinção. C. froelichii é aqui ilustrada pela primeira vez. Palavras-chave: Mata Atlântica, diversidade, Ericales, Neotrópico, Myrsinoideae. ABSTRACT: A rediscovered species of Cybianthus subgenus Cybianthus is described and illustrated. Cybianthus froelichii is most closely related to C. cuneifolius, but may be distinguished by its large leaves and sessile pistillate flowers. This species is endemic to the Atlantic Forest, Brazil, and is considered endangered. C. froelichii is illustrated here for the first time. Key words: Atlantic Forest, diversity, Ericales, Neotropic, Myrsinoideae. 1 Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Diretoria de Pesquisas, Rua Pacheco Leão 915, CEP 22460-030, Rio de Janeiro, RJ, Brasil. 2 Universidade Federal do Espírito Santo, Centro de Ciências Agrárias, Departamento de Biologia, Alto Universitário s.n., CEP 29500-000, Alegre, ES, Brasil. *Corresponding author. e-mail: [email protected] -

Network Scan Data
Selbyana 15: 132-149 CHECKLIST OF VENEZUELAN BROMELIACEAE WITH NOTES ON SPECIES DISTRIBUTION BY STATE AND LEVELS OF ENDEMISM BRUCE K. HOLST Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166-0299, USA ABSTRACf. A checklist of the 24 genera and 364 native species ofBromeliaceae known from Venezuela is presented, including their occurrence by state and indications of which are endemic to the country. A comparison of the number of genera and species known from Mesoamerica (southern Mexico to Panama), Colombia, Venezuela, the Guianas (Guyana, Suriname, French Guiana), Ecuador, and Peru is presented, as well as a summary of the number of species and endemic species in each Venezuelan state. RESUMEN. Se presenta un listado de los 24 generos y 364 especies nativas de Bromeliaceae que se conocen de Venezuela, junto con sus distribuciones por estado y una indicaci6n cuales son endemicas a Venezuela. Se presenta tambien una comparaci6n del numero de los generos y especies de Mesoamerica (sur de Mexico a Panama), Colombia, Venezuela, las Guayanas (Guyana, Suriname, Guyana Francesa), Ecuador, y Peru, y un resumen del numero de especies y numero de especies endemicas de cada estado de Venezuela. INTRODUCTION Bromeliaceae (Smith 1971), and Revision of the Guayana Highland Bromeliaceae (Smith 1986). The checklist ofVenezuelan Bromeliaceae pre Several additional country records were reported sented below (Appendix 1) adds three genera in works by Smith and Read (1982), Luther (Brewcaria, Neoregelia, and Steyerbromelia) and (1984), Morillo (1986), and Oliva-Esteva and 71 species to the totals for the country since the Steyermark (1987). Author abbreviations used last summary of Venezuelan bromeliads in the in the checklist follow Brummit and Powell Flora de Venezuela series which contained 293 (1992). -

La Minería Aurífera En El Parque Nacional Yapacana Amazonas Venezolano: Un Caso De Extrema Urgencia Ambiental Y Geopolítica, Nacional E Internacional
Foto Google Images La Minería Aurífera en el Parque Nacional Yapacana Amazonas Venezolano: Un caso de extrema urgencia ambiental y geopolítica, nacional e internacional Enero de 2019 “El interés que tiene el ELN por establecerse en esta zona es principalmente económico. Además del contrabando y la cocaína, también está la posibilidad de controlar la minería ilegal y las rentas derivadas del narcotráfico. ” (Eduardo Alvarez Vanegas y colaboradores1) “ ! Eso allá si que es bonito ! ” (minero entrevistado en Puerto Ayacucho, refiriéndose al orden y seguridad que impera en Yapacana impuesto por la guerrilla colombiana). 12018. Trayectorias y dinámicas territoriales de las disidencias de las FARC. Publicación de FUNDACIÓN IDEAS PARA LA PAZ. Abril de 2018. Serie Informes N. 30, Bogotá, Colombia CONTENIDO Siglas y Acrónimos ........................................................................................................................... I Resumen ......................................................................................................................................... II Introducción ................................................................................................................................... III Capitulo 1 ........................................................................................................................................ 1 Declaratoria y Contexto Jurídico ................................................................................................. 1 Referencias Citadas .................................................................................................................... -

The State of Venezuela's Forests
ArtePortada 25/06/2002 09:20 pm Page 1 GLOBAL FOREST WATCH (GFW) WORLD RESOURCES INSTITUTE (WRI) The State of Venezuela’s Forests ACOANA UNEG A Case Study of the Guayana Region PROVITA FUDENA FUNDACIÓN POLAR GLOBAL FOREST WATCH GLOBAL FOREST WATCH • A Case Study of the Guayana Region The State of Venezuela’s Forests. Forests. The State of Venezuela’s Págs i-xvi 25/06/2002 02:09 pm Page i The State of Venezuela’s Forests A Case Study of the Guayana Region A Global Forest Watch Report prepared by: Mariapía Bevilacqua, Lya Cárdenas, Ana Liz Flores, Lionel Hernández, Erick Lares B., Alexander Mansutti R., Marta Miranda, José Ochoa G., Militza Rodríguez, and Elizabeth Selig Págs i-xvi 25/06/2002 02:09 pm Page ii AUTHORS: Presentation Forest Cover and Protected Areas: Each World Resources Institute Mariapía Bevilacqua (ACOANA) report represents a timely, scholarly and Marta Miranda (WRI) treatment of a subject of public con- Wildlife: cern. WRI takes responsibility for José Ochoa G. (ACOANA/WCS) choosing the study topics and guar- anteeing its authors and researchers Man has become increasingly aware of the absolute need to preserve nature, and to respect biodiver- Non-Timber Forest Products: freedom of inquiry. It also solicits Lya Cárdenas and responds to the guidance of sity as the only way to assure permanence of life on Earth. Thus, it is urgent not only to study animal Logging: advisory panels and expert review- and plant species, and ecosystems, but also the inner harmony by which they are linked. Lionel Hernández (UNEG) ers. -

Pandon Ekamanin.Pdf
© Texto e ilustraciones: Hanneke Wagenaar © Fundación Editorial El perro y la rana, 2010 Centro Simón Bolívar Torre Norte, piso 21, El Silencio, Caracas - Venezuela, 1010. Teléfonos: (0212) 7688300 / 7688399. Correos electrónicos: [email protected] [email protected] Páginas web: www.elperroylarana.gob.ve www.ministeriodelacultura.gob.ve Edición al cuidado de: Rodolfo Castillo Elis Labrador Mónica Piscitelli Hecho el Depósito de Ley Depósito legal lf 40220108002277 ISBN 978-980-14-1076-8 IMPRESO EN VENEZUELA Hanneke Wagenaar Pandón ekamanín (Cuentacuentos) Al valle de Kamarata Nota de la ekamanín La primera idea que me asalta es que no son mis cuentos, son de los pemón, de su tradición oral, a ellos les pertenecen. La segunda idea es que, de ninguna manera, este trabajo tiene pretensiones antro- pológicas. Son sencillamente la razón para dibujar tepuyes, selvas y sabanas; arcoíris, nubes y cielos; personas que son pájaros y árboles que son personas; rocas parlantes, tucusitos en busca de una flor y acures que viajan dentro de troncos huecos en un mundo alucinado; anacondas gigantes y cerros que se abren para dibujar una tierra que me ha impactado quedándose pegada al cuerpo como una nueva capa de piel, utilizando los colores del cuero sutil de la boa tornasolada con la que los pájaros se cubrie- ron en el tiempo de Piá, cuando todo lo nombrable era humano. Es por ello -y con todo mi respeto- que escribo, a mi entender, los cuentos que escuché o leí en algún momento y que ahora les cuento a los karán, es decir, a los visitantes de otras latitudes y a los tüponkén de la ciudad. -

Floral Ontogeny and Vasculature in Xyridaceae, with Particular Reference to Staminodes and Stylar Appendages Author(S): M
Floral ontogeny and vasculature in Xyridaceae, with particular reference to staminodes and stylar appendages Author(s): M. Graça Sajo, Aline Oriani, Vera L. Scatena and Paula J. Rudall Source: Plant Systematics and Evolution, Vol. 303, No. 9 (November 2017), pp. 1293-1310 Published by: Springer Stable URL: https://www.jstor.org/stable/44853796 Accessed: 21-06-2021 11:00 UTC JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at https://about.jstor.org/terms Springer is collaborating with JSTOR to digitize, preserve and extend access to Plant Systematics and Evolution This content downloaded from 86.59.13.237 on Mon, 21 Jun 2021 11:00:03 UTC All use subject to https://about.jstor.org/terms Plant Syst Evol (2017) 303:1293-1310 CrossMark DOI 10. 1007/s00606-0 17- 1438-3 VOZ ORIGINAL ARTICLE Floral ontogeny and vasculature in Xyridaceae, with particular reference to staminodes and stylar appendages M. Graça Sajo1 • Aline Oriani1 • Vera L. Scatena1 • Paula J. Rudall2© Received: 29 March 2017 /Accepted: 24 June 2017 /Published online: 7 July 2017 © The Author(s) This2017. article is an open access publication Abstract We provide a detailed comparative evolutionary study historyof of the xyrid clade; this transition floral ontogeny and vasculature in Xyridaceae, occurred includ-either once followed by a reversal to fertile sta- ing Xyris , Abolboda and Orectanthe. -

Ve-Nr-01-Es.Pdf
MINISTERIO DEL AMBIENTE Y DE LOS RECURSOS NATURALES En el mes de junio de 1992, en el marco de la Conferencia de las Naciones Unidas sobre el Medio Ambiente y Desarrollo, Venezuela suscribió el Convenio sobre la Diversidad Biológica, el cual fue ratificado por el entonces Congreso de la República en el año 1994 por lo que es una ley aprobatoria. La aplicación de este Convenio significó un cambio conceptual al reconocer a los Estados sus derechos soberanos sobre los recursos biológicos, y al declarar la conservación de la diversidad biológica como patrimonio de la humanidad e impuso a las Partes contratantes un conjunto de obligaciones entre las que se encuentra la elaboración del “Informe de País” que hoy presentamos ante la Conferencia de las Partes. En este sentido, y atendiendo los lineamientos establecidos sobre el contenido y presentación del informe, se ha preparado un documento que incluye un estudio sobre la magnitud de la diversidad biológica presente en el país, que lo hace uno de los diez primeros países megadiversos del planeta condición que nos obliga en el conocimiento, conservación y uso sustentable de tan valioso recurso para el futuro de la humanidad. Consideramos importante resaltar que en la recién promulgada Constitución de la República Bolivariana de Venezuela se establece como una obligación de Estado la conservación y defensa de la Diversidad Biológica y a demás se reconoce el valor de los conocimientos ancestrales que poseen las comunidades indígenas, sobre la biodiversidad presente en las tierras que ocupan por lo que tendrán derecho a obtener beneficios derivados de la utilización de sus conocimientos tradicionales. -

Inflorescence Architecture and Floral Morphology of Aratitiyopea Lopezii (Xyridaceae) Lisa M
Aliso: A Journal of Systematic and Evolutionary Botany Volume 23 | Issue 1 Article 17 2007 Inflorescence Architecture and Floral Morphology of Aratitiyopea lopezii (Xyridaceae) Lisa M. Campbell New York Botanical Garden, Bronx Dennis Wm. Stevenson New York Botanical Garden, Bronx Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Campbell, Lisa M. and Stevenson, Dennis Wm. (2007) "Inflorescence Architecture and Floral Morphology of Aratitiyopea lopezii (Xyridaceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 23: Iss. 1, Article 17. Available at: http://scholarship.claremont.edu/aliso/vol23/iss1/17 Aliso 23, pp. 227–233 ᭧ 2007, Rancho Santa Ana Botanic Garden INFLORESCENCE ARCHITECTURE AND FLORAL MORPHOLOGY OF ARATITIYOPEA LOPEZII (XYRIDACEAE) LISA M. CAMPBELL1, 2 AND DENNIS WM.STEVENSON1 1The New York Botanical Garden, Bronx, New York 10458, USA 2Corresponding author ([email protected]) ABSTRACT Aratitiyopea lopezii is a robust perennial species of Xyridaceae from seasonally saturated, mid- to high-elevation, sandstone and granite sites in northern South America. The species lacks the scapose inflorescence characteristic of Xyridaceae and, having the gestalt of a rhizomatous bromeliad, it is seemingly aberrant in the family. However, closer examination confirms features consistent with the family and the previously noted morphological similarities to Orectanthe. Details of inflorescence structure and floral morphology are presented and compared to other genera of Xyridaceae. Key words: Aratitiyopea, Bromeliaceae, gynoecium appendage, inflorescence, Navia, nectary, Orec- tanthe, osmophore, pollen, Xyridaceae. INTRODUCTION florescence, and the few exceptions to this growth form (i.e., some Abolboda species, Achlyphila, and Aratitiyopea) have Aratitiyopea (Xyridaceae) is a monospecific genus of her- not been critically evaluated.