Journal of Basic and Applied Scientific Research (JBASR)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Reviewers (As Per the Published Articles) Year: 2017
List of Reviewers (as per the published articles) Year: 2017 Asian Research Journal of Mathematics ISSN: 2456-477X 2017 - Volume 2 [Issue 1] DOI : 10.9734/ARJOM/2017/30730 (1) Sami Ullah Khan, International Islamic University, Pakistan. (2) Mohammad Yaghoub Abdollahzadeh Jamalabadi, Dongguk University, Korea. Complete Peer review History: http://www.sciencedomain.org/review-history/17422 DOI : 10.9734/ARJOM/2017/29172 (1) Rashmi Awad, Devi Ahilya University, India. (2) Radosław Jedynak, Kazimierz Pulaski University of Technology and Humanities, Poland. Complete Peer review History: http://www.sciencedomain.org/review-history/17445 DOI : 10.9734/ARJOM/2017/30885 (1) Vasil G. Angelov, University of Mining and Geology, Bulgaria. (2) Emrullah Yasar, Uludag University, Turkey. Complete Peer review History: http://www.sciencedomain.org/review-history/17459 DOI : 10.9734/ARJOM/2017/29175 (1) Francesco Zirilli, Sapienza Universita Roma, Italy. (2) Jaya Bishwal, University of North Carolina at Charlotte, USA. (3) Radosław Jedynak, Kazimierz Pulaski University of Technology and Humanities, Poland. Complete Peer review History: http://www.sciencedomain.org/review-history/17460 DOI : 10.9734/ARJOM/2017/31160 (1) Golam Hafez, Chittagong University of Engineering and Technology, Chittagong-4349, Bangladesh. (2) Horácio Santana Vieira, Universidade Federal da Paraíba, Brazil and Universidade Estadual da Paraíba, CEP, Brazil. Complete Peer review History: http://www.sciencedomain.org/review-history/17499 2017 - Volume 2 [Issue 2] DOI : 10.9734/ARJOM/2017/30764 (1) U. S. Mahabaleshwar, Government First Grade College for Women, India. (2) Jagdish Prakash, University of Botswana, Botswana. (3) Humaira Yasmin, Majma’ah University, Saudi Arabia. Complete Peer review History: http://www.sciencedomain.org/review-history/17513 DOI : 10.9734/ARJOM/2017/31152 (1) Francisco Bulnes, Tescha, Mexico. -

For Facile Photodegradation of Methyl Orange Dye
Desalination and Water Treatment 223 (2021) 403–413 www.deswater.com May doi: 10.5004/dwt.2021.27137 TiO2 subsidized periodic mesoporous organosilicate (TiO2@PMOS) for facile photodegradation of methyl orange dye Khurram Shahzada, Muhammad Imran Khanb,*, Shabnam Shahidac, Abdallah Shanablehb, Noureddine Elboughdirid,e, Shazia Jabeenf, Suryyia Manzoorg, Shagufta Zafarh, Hassan Alii, Abdul Waheed Rabbanij, Javier Fernandez-Garciak, Aziz ur Rehmana,* aDepartment of Chemistry, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan, emails: [email protected] (A. ur Rehman), [email protected] (K. Shahzad) bResearch Institute of Sciences and Engineering, University of Sharjah, Sharjah 27272, United Arab Emirates, emails: [email protected] (M. Imran Khan), [email protected] (A. Shanableh) cDepartment of Chemistry, University of Poonch, Rawalakot, Azad Kashmir, Pakistan, email: [email protected] dChemical Engineering Department, College of Engineering, University of Ha’il, P.O. Box: 2440, Ha’il 81441, Saudi Arabia, email: [email protected] eChemical Engineering Process Department, National School of Engineering Gabes, University of Gabes, Gabes 6011, Tunisia fDepartment of Environmental Sciences, The Women University of Multan, Multan 60000, Pakistan, email: [email protected] gInstitute of Chemical Sciences, Bahauddin Zakariya University, Multan 60800, Pakistan, email: [email protected] hDepartment of Chemistry, The Government Sadiq College Women University, Bahawalpur 63000, Pakistan, -

Comparative and Qualitative Study of Fruit Leather from Wild and Cultivated
Pure Appl. Biol., 9(4): 2279-2284, December, 2020 http://dx.doi.org/10.19045/bspab.2020.90242 Research Article Comparative and qualitative study of fruit leather from wild and cultivated apricot (Prunus armeniaca L.) grown under agro-climatic condition of Rawalakot Azad Kashmir Pakistan Zamird Bashir1, Imtiaz Hussain1, Nadra Khan2*, Saiqa Bashir1, Nosheen Bashir3, Nagina Rafique1 and Shafiq Ur Rehman4 1. Department of Food Science and Technology, Faculty of Agriculture, University of the Poonch Rawalakot, 12350, AJK-Pakistan 2. Department of Horticulture, Faculty of Agriculture, University of the Poonch Rawalakot, 12350, AJK-Pakistan 3. Department of Veterinary, the University of Poonch Rawalakot, 12350, AJK-Pakistan 4. Department of Plant breeding and genetics, Faculty of Agriculture Sciences, University of the Poonch, Rawalakot-Pakistan *Corresponding author’s email: [email protected] Citation Zamird Bashir, Imtiaz Hussain, Nadra Khan, Saiqa Bashir, Nosheen Bashir, Nagina Rafique and Shafiq Ur Rehman. Comparative and qualitative study of fruit leather from wild and cultivated apricot (Prunus armeniaca L.) grown under agro-climatic condition of Rawalakot Azad Kashmir Pakistan. Pure and Applied Biology. Vol. 9, Issue 4, pp2279-2284. http://dx.doi.org/10.19045/bspab.2020.90242 Received: 13/03/2020 Revised: 16/06/2020 Accepted: 25/06/2020 Online First: 08/07/2020 Abstract There is an abundant apricot fruit production in Rawalakot Azad Jammu and Kashmir, Pakistan. Unfortunately due to improper post-harvest management practices, more than 50 % of post-harvest losses have been recorded annually. The seasonal fruit production offers an excellent opportunity for the development of novel fruit products. The current study was planned to develop the pulp products of apricot with an objective to minimize the post-harvest losses of local apricot and to evaluate the physico-chemical and sensory properties of fruit leather and pulp. -

CURRICULUM VITAE Dr. Muhammad Akram (Associate Professor)
CURRICULUM VITAE Dr. Muhammad Akram (Associate Professor) Chairperson, Department of Eastern Medicine, Directorate of Medical Sciences, Faculty of Life Sciences, Government College University Faisalabad-Pakistan Ex-Chairman, Department of Eastern Medicine, Faculty of Medical & Health Sciences, University of Poonch, Rawalakot Azad Kashmir, Pakistan. Coordinator, M. Phil and PhD in Eastern Medicine, Directorate of Medical and Health Sciences, Government College University Faisalabad, Pakistan CORRESPONDENCE DETAILS Contact Address: Department of Eastern Medicine, Directorate of Medical Sciences, Faculty of Science and Technology, Government College University Faisalabad. Permanent Address: Moza Nothain, Post office Chak Mubarak, Thana Bhera, Tehsil Bhalwal, District Sargodha Tel: Off: 0092-042-9200127: Res: (Mobile) 0092-0334-3367632; 0092-345-2888394; 0092- 0305-2049573 WhatsApp:+92-3452888394, IMO:+92-3452888394, Skype:hucon2008, Facebook:[email protected] Google scholar: [email protected] Researchgate: [email protected] Researcher ID:A-5679-2018 ORCID:0000-0001-7863-8803 E-mail: [email protected] ; [email protected] PERSONAL INFORMATION Name: Muhammad Akram Father Name: Muhammad Khan Date of Birth: February 12, 1980 Domicile: Sargodha, Punjab, Pakistan Religion: Islam National Identity Card No:42201-4270587-9 Qualification: B.E.M.S, M. Phil, Ph.D Area of Specialization: Eastern Medicine (Xanthine Oxidase Inhibition by Plants Extract and Clinical Efficacy of Herbal Formulation in Gouty Arthritis) Carrier Objective My objective is to be a part of an organization, which values merits, research skills, respects hard work and which can utilize my qualification and abilities for the mutual growth.And to remain embarked on the challenging field of eastern medicine where experience can be leveraged, knowledge and skills can be enhanced and humanity can be served in best possible way. -

Exploration of Exotic Ethno Botanical Potential in District Poonch Azad
International Journal of Herbal Medicine 2019; 7(6): 05-09 E-ISSN: 2321-2187 P-ISSN: 2394-0514 IJHM 2019; 7(6): 05-09 Exploration of exotic ethno botanical potential in Received: 04-09-2019 Accepted: 08-10-2019 district Poonch Azad Jammu Kashmir Sardar Irfan Mehmood Department of Botany, Govt. Sardar Irfan Mehmood, Nuzhat Shafi, Zeenat Jannat, Shabnum Shahida, Boys PGC College, Poonch Azad, Abbaspur, Kashmir, Jammu and Sajid Majeed and Tariq Habib Kashmir, India Abstract Nuzhat Shafi Azad Jammu and Kashmir is a famous natural territory with ecological zones for medicinal and aromatic Department of Zoology, plants. Especially district Poonch is blessed with wide range of climatic zones from subtropical to alpine University Muzaffarabad Azad, zones which provide a home for medicinal herbs. Keeping these facts in mind twelve exotic species Kashmir, Jammu and Kashmir, (Calendula officinalis L., Cynara scolymus L., Kalanchoe pinnata (Lam.) Pers., Lavendula angustifolia India Mill., Mimosa pudica L. Mentha X piperita L., Melissa officinalis L. Origanum majurona L. Rosmarinus Zeenat Jannat, officinalis L., Stevia rebaudiana (Bertoni), Solanum muricatum Ait., Thymus vulgaris L. were acquired Department of Zoology, from (Bio Conservation Institute) NARC Islamabad and were planted in botanical repository at Govt. University of Poonch Rawalakot Boys College Campus. After successful plantation and cultivation different formulations like powder, oil, Azad, Kashmir, Jammu and paste, infusion and extract were prepared from these medicinal plants. To check medicinal value of each Kashmir, India species these formulation were distributed to 100 local habitants of Azad Kashmir. These investigations on exotic species in local area were continued during the last four years. -

Sr# University Focal Person with Contacts 1 University of Balochistan
Sr# University Focal person with contacts 1 University of Balochistan, Quetta Mr. Abdul Malik, [email protected], [email protected] Ph: 081-9211836 & Fax# 081-9211277 AmanUllah Sahib (Principal Law College) 2 BUITEMS, Quetta Ms. Kinza (Manager Financial Assistance) [email protected] 3 Sardar Bhadur Khan Women Ms. Huma Tariq (Assistant Registrar Financial Aid Office) University, Quetta [email protected]; Ph:0819213309 4 University of Loralai Mr. Noor ul Amin Kakar (Registrar) [email protected] 5 University of Turbat, Turbat Mr. Ganguzar Baloch (Deputy Registrar) [email protected] 6 Balochistan University of Engr. Mumtaz Ahmed Mengal Engineering & Technology Khuzdar [email protected] Ph: 0848550276 7 Lasbela University of Water & Haji Fayyaz Hussain(UAFA) Marine sciences, Lasbela [email protected] Office: Ph: 0853-610916 Dr.Gulawar Khan [email protected] 8 National University of Modern Prof. Usman Sahib (Regional director) Languages (NUML), Quetta Campus [email protected]; [email protected] Ph: 081-2870212 9 University of Peshawar, Peshawar Mr. Fawad Khattak (Scholarship Officer) [email protected], Ph: 091-9216474 10 Khyber Medical University, Mr. Fawad Ahmed (Assistan Director Admissions) Peshawar [email protected] Ph: 091-9217703 11 Islamia College, Peshawar Mr.Sikandar Khan (Director Students Affairs) [email protected] 12 Kohat University of science and Mr. Zafar Khan (Director Finance) Technology(KUST), Kohat [email protected], Rahim Afridi (Dealing Assistant)[email protected] 13 University of Agriculture, Peshawar Prof. Dr. Muhammad Jamal Khan [email protected]; [email protected] 14 University of Engineering & Mr. Nek Muhammad Khan (Director Finance/Treasurer) Technology, Peshawar [email protected] Ph: 091-9216497 15 IM Sciences, Peshawar Mr. -

Pdf 808.99 K
Journal of Medicinal and Chemical Sciences 4 (2021) 75-83 Journal of Medicinal and Chemical Sciences Journal homepage: www.jmchemsci.com Original Article Prevalence and Treatment of Hypertension in District Bhimber, Azad Jammu and Kashmir Nargis Rehman1, Mr Izharullah2, Jawad Zaheer2, Muhammad Akram3*, Suresh Ghotekar4*, Muhammad Idrees Khan3 1Department of Eastern Medicine and Surgery, Faculty of Medical and Health Sciences, University of Poonch, Rawalakot, Azad Jammu and Kashmir, Pakistan 2Department of Pharmacy, Faculty of Medical and Health Sciences, University of Poonch, Rawalakot, Azad Jammu and Kashmir, Pakistan 3Department of Eastern Medicine, Government College University, Faisalabad, Pakistan 4Department of Chemistry, Sanjivani Arts, Commerce and Science College, Kopargaon 423 603, Savitribai Phule Pune University, Maharashtra, India A R T I C L E I N F O A B S T R A C T Article history Hypertension is defined as a persistent elevation in blood pressure. Hypertension is also called as silent killers as mostly it is asymptomatic. : Received 2020-11-20 Hypertension can be due to increased heart rate and blood volume. Received in revised: 2020-12-26 Hypertension is also due to elevated peripheral vascular arteriolar smooth Accepted: 2020-12-28 muscle pitch. Major signs and symptoms of hypertension are blurred vision, Manuscript ID: JMCS-2011-1138 confusion, irregular heartbeat and shortness of breath. Hypertension is classified into two major categories including, primary hypertension and secondary hypertension. Hypertension complications are hemorrhage, DOI:10.26655/JMCHEMSCI.2021.9 atherosclerosis, renal artery stenosis, angina pectoris, myocardial infarction, K E Y W O R D S and retinopathy. Estimated global prevalence of hypertension is high long term projections suggested that by 2025 (29%) of adult worldwide are Hypertension suffering from hypertension (1.56 billion). -

Nexus Between Political Instability and Economic Growth in Pakistan
Available online at www.sciencedirect.com ScienceDirect Procedia - Social and Behavioral Sciences 230 ( 2016 ) 325 – 334 3rd International Conference on New Challenges in Management and Organization: Organization and Leadership, 2 May 2016, Dubai, UAE Nexus between Political Instability and Economic Growth in Pakistan Aftab Hussain Tabassama, Shujahat Haider Hashmib,*, Faiz Ur Rehmanc aLecturer, University of Poonch, Rawalakot, AJK, Pakistan bLecturer, CUST, Islamabad, Pakistan cDirector, Institute of Kashmir Studies, AJK University, Pakistan Abstract This study has explored the effect of political unrest on economic of Pakistan and its volatility over the period of last 22 years using annual time series data, which have been further decomposed into different quarters to capture interim effects. Terrorism, election, regime and strikes have been used as political instability proxies. ARCH and GARCH models have been used to examine the outcome of political uncertainty on the economic progress, that is, GDP in Pakistan. From the outcomes of GARCH (1, 1) model through the independent variables in the mean equation, it was found that among terrorism, election, regime and strikes, only terrorism has significant negative effect on the mean equation of the dependent variable. The results of GARCH (1, 1) model with independent variables in the variance equation shows that elections and regimes have significant negative effect on volatility of GDP. The overall results imply that political instability has significant negative effect on economic growth and the government should take corrective measures to bring political stability. © 2016 TheThe Authors. Authors. Published Published by by Elsevier Elsevier Ltd. Ltd. This is an open access article under the CC BY-NC-ND license Peer-review(http://creativecommons.org/licenses/by-nc-nd/4.0/ under responsibility ofthe Ardabil Industrial). -

Curriculum Vitae
CURRICULUM VITAE Name: Imran Ali Mobile #: +923464048255 Passport No: TU1799831 Office Phone: 92-05827-961999 Fax: 92-05827-961054 E-mail: [email protected] Address: Department of Biotechnology, Mirpur University of Science and Technology (MUST), Mirpur-10250, (AJK), Pakistan 1. EDUCATIONAL BACK GROUND Year Degree Subject Institution/ University 2006 M.Phil Mol. Bio. NCEMB, University of the Punjab, Lahore, Pakistan 2003 B.Sc.(Hons.) Agri. University of AJ&K, Azad Kashmir, Pakistan 1998 F.Sc. Premed B.I.S.E. Sargodha, Pakistan 1995 Matric Science B.I.S.E. Mirpur Azad Kashmir, Pakistan. 2. PROFESSIONAL EXPERIENCE 14/05/2012- Present Assistant Professor (BPS-19) Department of Biotechnology Mirpur University of Science and Technology (MUST) Mirpur, 10250 (AJK), Pakistan 23/02/2011- 14/05/2012 Lecturer (BPS-18) Department of Biotechnology Mirpur University of Science and Technology (MUST) Mirpur, 10250 (AJK), Pakistan 01/03/2008-01/03/2011 Research Officer (BPS-17) School of Biological Sciences University of the Punjab, Lahore, Pakistan 01/01/2006 – 31/12/2007 Research Officer (BPS-17) National Centre of Excellence in Molecular Biology University of the Punjab, Lahore, Pakistan M. Phil. Students Supervised: i. Waqar Haider (2012). Antihyperlipidemic Potential of Solanum nigrum Aerial Parts Extract in Lipofundin Induced Hyperlipidemic Rabbits. ii. Aimen Saeed (2012). The Study of Antihyperlipidemic Potential of Solanum xanthocarpum in Lipofundin Induced Hyperlipidemic Rabbits. iii. Faiza Bashir (2013). Heterologous Expression of Endoglucanase (Cel5S) of Bacillus Pumilus MUST-3 in Escherichia coli BL-21 iv. Haseena Rasheed (2013). Recombinant Expression of Alpha Amylase From Bacillus subtilis MUST-2 in Escherichia coli BL-21 (CodonPlus). -
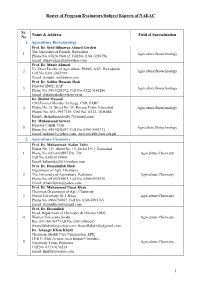
Roster of Program Evaluators/Subject Experts of NAEAC
Roster of Program Evaluators/Subject Experts of NAEAC Sr. Name & Address Field of Specialization No 1. Agriculture Biotechnology Prof. Dr. Syed Dilnawaz Ahmed Gerdezi The University of Poonch, Rawalakot 1 Agriculture Biotechnology Phone No. 05824-960012, Cell No. 0301-5280796 Email: [email protected] Prof. Dr. Munir Ahmad Ex. Dean Faculty of Agriculture, PMAS, AAU, Rawalpindi 2 Agriculture Biotechnology Cell No. 0301-5055989 Email: [email protected] Prof. Dr. Safdar Hussain Shah Director IBGE, UAP 3 Agriculture Biotechnology Phone No. 091-9221072, Cell No. 0322-5164286 Email: [email protected] Dr. Shahid Masood CSO/Former Member Incharge, CSD, PARC 4 House No. 21. Street No. 29, Korang Town, Islamabad Agriculture Biotechnology Phone No. 051-5957329, Cell No. 0333-7405084 Email: [email protected] Dr. Muhammad Sarwar Director CABB, UAF 5 Agriculture Biotechnology Phone No. 041-9201087, Cell No. 0300-5551731 Email: [email protected], [email protected] 2. Agriculture Chemistry Prof. Dr. Muhammad Kalim Tahir House No. 119, Street No. 19, Sector I-9/1, Islamabad 1 Phone No. 051-4430597 Ext. 724 Agriculture Chemistry Cell No. 0345-5157460 Email: [email protected] Prof. Dr. Hamidullah Shah Department of Agri. Chemistry 2 The University of Agriculture, Peshawar Agriculture Chemistry Phone No. 091-9216903, Cell No. 0300-5935576 Email: [email protected] Prof. Dr. Muhammad Umar Khan Chairman Department of Agri. Chemistry 3 Gomal University, D. I. Khan Agriculture Chemistry Phone No. 0966750087, Cell No. 0346-8991765 Email: [email protected] Prof. Dr. Ihsanullah Head, Department of Chemistry & Director ORIC, 4 Women University Swabi Agriculture Chemistry Res: 091-5619637 Cell No. -

Ethnopharmacological Studies of Indigenous Plants in Kel Village
Ahmad et al. Journal of Ethnobiology and Ethnomedicine (2017) 13:68 DOI 10.1186/s13002-017-0196-1 RESEARCH Open Access Ethnopharmacological studies of indigenous plants in Kel village, Neelum Valley, Azad Kashmir, Pakistan Khawaja Shafique Ahmad1*, Abdul Hamid2, Fahim Nawaz3, Mansoor Hameed4, Farooq Ahmad4, Jiabin Deng5, Noreen Akhtar6, Ambreen Wazarat1 and Sehrish Mahroof1 Abstract Background: This explorative study was undertaken for the first time in Kel village located in the Upper Neelum Valley, Azad Kashmir, Pakistan. The purpose was to document the indigenous knowledge of the native people used in the preparation of herbal medicines. Methods: To get the data on traditional uses of medicinal plants, 20 informants were interviewed. Quantitative ethnobotanical indices, i.e., use value (UV), relative frequencies of citation (RFC), informant consensus factor (Fic), fidelity level (FL), data matrix ranking (DMR), preference ranking (PR), and jaccard index (JI), were calculated for the recorded medicinal plants. Results: A total of 50 medicinal plants belonging to 33 families used in 13 disease categories were documented. Leaves were the frequently used plant parts, and decoction was the commonly used method for herbal medicine. Plants with high use value were Berberis lycium (2.05), Impatiens glandulifera (1.95), Artemisia scoparia (1.75), Ageratum conozoides (1.75), and Achillea millefolium (1.7). The highest RFC value was calculated for Berberis lycium (0.75), Cynoglossum lanceolatum (0.65), and Impatiens glandulifera and Achillea millefolium (0.60 each). The maximum informant consensus factor was for urinary system, cardiac diseases, baldness, and abortion and miscarriage (1.00). Berberis lyceum (95%) used in jaundice, hepatitis, typhoid, fever, and tuberculosis disorders. -

Effects of Seeding Time and Weed Control Methods in Direct Seeded Rice (Oryza Sativa L.)
Mubeen et al., The Journal of Animal & Plant Sciences, 24(2): 2014, Page:J.5 Anim.34-542 Plant Sci. 24(2):2014 ISSN: 1018-7081 EFFECTS OF SEEDING TIME AND WEED CONTROL METHODS IN DIRECT SEEDED RICE (ORYZA SATIVA L.) K. Mubeen, M. A. Nadeem*, A. Tanveer* and A. J. Jhala** Department of Agronomy, University of Poonch, Rawalakot, Pakistan *Department of Agronomy, University of Agriculture, Faisalabad, Pakistan **Department of Agronomy and Horticulture, University of Nebraska-Lincoln, Keim Hall, Lincoln, NE 68583, USA Corresponding Author E-mail: [email protected] ABSTRACT Field experiments were conducted during the 2 year period from 2008 and 2009 to determine the effects of three seeding dates and seven weed control methods in DSR. The results suggested that seeding in the first week of July reduced weed density and biomass, increased kernel weight, leaf area index, and kernel yield compared to seeding rice in the first week of July. Amongst weed control methods, penoxsulam followed by hand-hoeing at 30 days after seeding (DAS) reduced weed density as low as ≤ 6 and ≤ 28 plants m-2 at 35 DAS and at harvest, respectively during both the years which was comparable with hand-hoeing at 15, 30 and 45 DAS. In addition, rice yield attributes including number of tillers, kernel weight, leaf area index, leaf area duration were higher, while kernel yield in this treatment was 70 and 61% higher compared to nontreated control during 2008 and 2009, respectively. A foliar spray of sorghum and sunflower water extract at 20 and 40 DAS and sorghum mulch at 6 t ha-1 were effective for weed control and secured kernel yield respectively, > 33% and 27% higher compared with the non-treated control.