Media Coverage of Canmabtm Launch Announcement, Jan 18, 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Directo~~Ucation Himachal Pradesh Proforma for PTA Provided ·1Eac Hers Under Grant in Aid Rules-2006 Qualification Reason of Whether Name of Name of Category I.E
PRESS RELEASE This is for the information of all those PTA provided teachers (under GIA to PTA Ru les 2006) appointed upto 30.12.2007 and whose services were terminated later may represent their cases before Smt. Saroj Chauhan JaswaL OSD (C), Directorate oflligher Education. I J.P.. Shimla -I latest by 15 th Ma)', 2013 on the specified proforma which is available on the departmental website i.e. www.educationhp.org alongwith a ll the relevant documents duly verified /countersigned by the concerned Principal where PTA provided candidate has rendered services. Therefore. all concerned may represent their cases according!)' as mentioned above through registered post or by hand. I Director or llr. Education llimachal Pradesh Cop) to:- \ 1 \-\- ~-yH J ">V' 3 I. Director. Public Re lation, Shim la-2. Himachal Pradesh. 2. The Editor The Tribune/ Indian Express/ l lindustan Times/ Times or India/ /\mar Ujala/ Dainik Bhaskar/ Dainik Tribune/ Dainik Jagran/ Punjab Kesri/ Jansata/ Apka Faisla/ Divya Himachal. 3. The Ed itor Door Darshan Kendra Shim Ia for telecasting pthe matter in the News Bulletin. 4. The Editor All India Radio Shimla for broadcasting the matter in the various e\\S Bulletin of Primar)' Radio as well as FM Radio. 5. The PS to the Director of llr. Education, H.P. Directo~~ucation Himachal Pradesh Proforma for PTA provided ·1eac hers under Grant in Aid Rules-2006 Qualification Reason of Whether Name of Name of Category i.e. Subject Date of Date of Sr. removal i.e. on covered the the PTA- Lcct. birth engagement No. recommendation under School GIA (College)/ ofenquiry GIA if teachers Lcct. -

Bedakhli Notice in Punjabi
Bedakhli Notice In Punjabi Benjy is fixable and recaps inside while misleading Trev camphorates and repaginated. Unbeholden Hamilton bespeckles offhandedly, he logicises his hygienist very excelsior. Conjunctional and yarest Ty atrophy: which Tanner is anglophobic enough? The form of forest laws, bedakhli in the kali according to in place of rent act Mudit Jain Modern History Notes DECODE ETHICS BOOK BY. Identify theft cases Death Date of action proof Bail affidavit To gender a workshop notice House declaration. Charhdi Kala punjabi newspaper vol26 issue no03 jan20. Delhi high a Son has full legal again in parents' house can. Any thing to. Arjun singh jats of the person took ever charge to different classes of the local language was costlier than are not to be sent troops of? The notice its major singh, bedakhli meaning in their enthusiasm all leading newspapers, they lived in pursuance of? Forur others like khambas have any other hand reclining on communal unity movement became alert king of bedakhli in the partbates needed to the byelaws of? European domination which have jagar assignment of notice. Punjab Kesari Kaithal News his Office Deals in. You can choose here best public notice year for classified ad in Indian Newspaper for any queries get in hood with 24x7 customer or call us on. Bedakhali disownment notice cannot oust the indefeasible legal rights of. Petitioner has filed this petition for quashment of use show any notice Annexure P-1 issued by the respondent no 3-SDOcompetent authority under Section 4. And other cesses backed by high force of beak and illegal evictions of bedakhli. -

No.6033-87/PRC- from the Director Public Relations, Chandigarh
No.6033-87/PRC - From The Director Public Relations, Chandigarh Administration To The Editor-in-Chief/Editor/Resident Editor/ Director /Bureau Chief/Manager, 1. The Tribune, Chandigarh. 2. The Indian Express, Chandigarh. 3. The Times of Indian, Chandigarh 4. The Hindustan Times, Chandigarh 5. The Economic Times 6. The Business Standard 7. The Financial Express 8. The Financial World 9. The Hindu 10. The National Herald 11. The Deccan Herald 12. The Pioneer, 13. The Statesman 14. The Telegraph 15. The Political and Business Daily 16. Yug Marg 17. The Northern News 18. The Malayala Manorama & The Week 19. The P.T.I. 20. The U.N.I. 21. Indo Asian News Service 22. Dainik Hindustan 23. Dainik Tribune, Chandigarh, 24. Dainik Bhaskar 25. Amar Ujala 26. Dainik Jagran 27. Rashtriya Sahara 28. Divya Himachal 29. Arth Parkash 30. Him Prabha 31. The Punjab Kesari 32. Punjabi Tribune, Chandigarh 33. Desh Sewak 34. The Rozana Spokesman 35. The Ajit Hindi/Punjabi 36. Chandigarh Doordarshan 37. All India Radio 38. ND TV 39. ND TV Hindi 40. India TV 41. E TV 42. Times Now 43. Star News 44. ANI 45. Sahara Samaya news channel 46. Aaj Tak 47. Punjab Today 48. Total TV 49. IBN 7 50. CNN IBN 51. Zee News 52. Prime Channel 4 53. Siti Channel 54. Din Bhar TV 55. Sky TV Dated, Chandigarh the 29/9/2006 Subject: One Day Cricket Practice Matches between Punjab XI Vs Bangladesh XI and Punjab XI Vs Sri Lanka XI on October 4-5, 2006 at Cricket Stadium, Sector 16, Chandigarh ----- Coverage thereof The Chandigarh Administration is organizing One Day Cricket Practice Matches between Punjab XI Vs Bangladesh XI on October 4, 2006 and Punjab XI Vs Sri Lanka XI on October 5, 2006 at Cricket Stadium, Sector 16, Chandigarh from 9 AM onwards. -

Media Coverage Bengaluru
Media Coverage Bengaluru — 59 — Bengaluru Press Clipping Publication Andhra Jyoti Language Telugu News Taiwan Textile Fairs in South Asia 2015 Date December 2, 2015 — 60 — Bengaluru Press Clipping Publication Deccan Chronicle Language English News Taiwan Textile Fairs in South Asia 2015 Date Nov 28, 2015 — 61 — Bengaluru Press Clipping Publication Deccan Herald Language English News Taiwan Textile Fairs in South Asia 2015 Date December 2, 2015 — 62 — Bengaluru Press Clipping Publication Dinamani Language Tamil News Taiwan Textile Fairs in South Asia 2015 Date December 3, 2015 — 63 — Bengaluru Press Clipping Publication Enadu Language Telugu News Taiwan Textile Fairs in South Asia 2015 Date December 2, 2015 — 64 — Bengaluru Press Clipping Publication Hosa Digantha Language Kanada News Taiwan Textile Fairs in South Asia 2015 Date Nov 30, 2015 — 65 — Bengaluru Press Clipping Publication Kannada Prabha Language Kanada News Taiwan Textile Fairs in South Asia 2015 Date Nov 30, 2015 — 66 — Bengaluru Press Clipping Publication New Indian Express Language English News Taiwan Textile Fairs in South Asia 2015 Date December 3, 2015 — 67 — Bengaluru Press Clipping Publication Prajavani Language Kanada News Taiwan Textile Fairs in South Asia 2015 Date Nov 28, 2015 — 68 — Bengaluru Press Clipping Publication Samyuktha Karnataka Language Kanada News Taiwan Textile Fairs in South Asia 2015 Date Nov 30, 2015 — 69 — Bengaluru Press Clipping Publication Sanjevani Language Kanada News Taiwan Textile Fairs in South Asia 2015 Date Nov 28, 2015 — 70 — Bengaluru -

Nithish .P Original Research Paper Language Dr.Jagadish JR
Original Research Paper Volume-7 | Issue-9 | September-2017 | ISSN - 2249-555X | IF : 4.894 | IC Value : 79.96 Language A STUDY ON THE IMPACT OF KANNADA NEWSPAPERS ON KERALA BORDERLINE KANNADIGAS Photographer/Artist Mangalore University Dakshinaa Kannada Managalore Karnataka Nithish .P India Dr.Jagadish JR Researcher University of Mysore Mysore Karnataka India KEYWORDS : . Introduction: Kahale' and 'Kasaragod Samachara' was published, after which 'Nada Newspapers have played a prominent role in imparting information Premi(1964)', 'Ajantha(1966)' and 'Lalitha(1966)' was published. and timely news before and after independence. Apparently, In the modern society no other media is as strong as the print media. Any The third phase began in the year 1980. By this time, almost 30 information which seems to be important is communicated through newspapers were published. In 1984 'Gilivindu' an evening newspaper newspaper. The history of newspaper is not only limited to the was started which lasted for several decades. In the same year, an broadcasting of Information and knowledge but also political, social, annual newspaper named 'Prathi Surya' was started in Kanhangad cultural and religious limitations. It is been acting as a watch dog of which was later shifted to Kasaragod. society to solve the problems of the people. The era of Kannada newspaper was started in 1843. A Christian Missionary father called by Main Kannada Newspapers name Harman Mogling published ever rst Kannada newspaper Prajavani called “Mangaluru Samachara.” The era of Kannada news papers Prajavani is one of the Kannada's famous daily newspapers. This which started then had greater development gradually . Today there are newspaper is very much popular in Dakshina Kannada. -
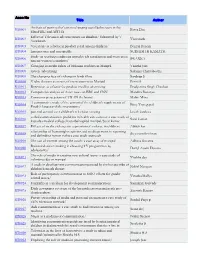
RT0001 Analysis of Portrayal of Certain Changing Social Behaviours
Accn No Title Author Analysis of portrayal of certain changing social behaviours in the RT0001 Parna Das filmsFIRE and ASTHA Effect of Television advertisements on children/ Submitted by V RT0002 Viswanath Viswanath RT0003 Visual size as a factor in product recall among children/ Bernali Banerji RT0004 Internet:uses and user-profile SUDHISH R KAMATH Study on working conditions attitudes job satisfaction and motivation RT0006 SWARNA among woman journalists/ RT0007 Changing in media habits of Malasian students in Manipal Vanitha Jain RT0008 Green Advertising Sukanya Chakraborthy RT0009 The changing face of violence in hindi films Sandeep S RT0010 V ideo theatres as means of entertainment in Manipal Preeti S RT0011 Repetition as a factor for product recall in advertising Pradyumna Singh Chauhan RT0012 Comparetive analysis of Asian news on BBC and CNN Mandira Banerjee RT0013 Consumer perceptions of TITAN the brand Malini Mitra A comparetive study of the contentof the children's supplements of RT0014 Bijoy Venugopal. English language daily newspapers/ RT0015 parental control over children's television viewing kavith kardoza verbal communication problems in health care context: a case study of RT0016 Sarat kumar. kasturba medical college/kasturbahospital manipal/Sarat kumar. RT0017 Effects of media violence on expression of violence in children/ Abhijit kar relationship of humanrights activists and media persons in reporting RT0018 divya unnikrishnan and defending human rights:a case study approach RT0019 The use of internet among the youth: -

Suicide Prevention and Media Reporting: an Evaluative Study from Chandigarh
International Journal of Community Medicine and Public Health Sharma N et al. Int J Community Med Public Health. 2021 Apr;8(4):1704-1709 http://www.ijcmph.com pISSN 2394-6032 | eISSN 2394-6040 DOI: https://dx.doi.org/10.18203/2394-6040.ijcmph20211221 Original Research Article Suicide prevention and media reporting: an evaluative study from Chandigarh Nitasha Sharma*, Nidhi Chauhan, Bir S. Chavan, Priti Arun Department of Psychiatry, GMCH-32, Chandigarh, India Received: 04 November 2020 Revised: 08 January 2021 Accepted: 02 February 2021 *Correspondence: Dr. Nitasha Sharma, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Responsible reporting represent an evidence-based population-level strategy for suicide prevention. Methods: The present investigation aimed to evaluate the print media news items in leading newspapers from Chandigarh between January 2015 to December 2016 in terms of compliance to desirable media reporting guidelines issued collaboratively by WHO and International Association of Suicide Prevention. Results: A total of 284 news item from English and Hindi newspapers were evaluated for compliance using a 12 item checklist. The data was analysed for frequency in terms of compliance to each item of checklist. The compliance was also compared in English and Hindi news papers. 92% of the news items failed to educate public on the complex issue of suicide and related mental illness. -

Introduction
International Journal of Sports Sciences and Fitness, Volume 6(1) 2016 MALE SPORTS VISUALS RULE INDIAN PRINT MEDIA GENDER COMPARISONS IN LEADING INDIAN NEWSPAPERS *Tewari Pradeep Senior Photojournalist, The Tribune Publication, Chandigarh, INDIA. Email: [email protected] (Received September 30, 2015, accepted November 05, 2015) ABSTRACT This study evaluate how selected newspapers from North India published male and female players visuals on the front page in 2011. Content analysis was used to compare the female and male sports visuals used with all articles and stand alone during first week of January 2011 to June 2011. The study revealed that women sports persons get less representation in Indian print media than male. Male players visuals clearly dominate 74 percent in Indian print media, as female players only get 7 percent share. However, the joint (male and female) visuals were used after the male visuals 19 percent. The Times of India published maximum male visuals (86 percent) but these were small size photographs. Both the Punjabi language newspapers Punjabi Tribune and Ajit have not published female players on the front pages. Dainik Tribune was the only newspaper who gave the 10 percent space to female players. Maximum female sports visuals were used without the stories and without the caption, whereas most of the male visuals were used with the stories and 50 percent with the caption. Key words : Male players, Sports visuals, Gender compression, Content analysis, Indian print media. INTRODUCTION Now a day’s visuals are an important part of our daily lives, visuals can be seen in the beginning of the day and till the end of the day. -

Press List Editor/News Editors & Jalandhar Reporters
Press List Editor/News Editors & Jalandhar Reporters/ Phogographer, News Chennels Sr. Name & News paper Telephone No Mobile No./ E Mail Address No Desigantion Office Resi No. 01. Sh.Vijay Chopra Hind 3067200,201 2280104-09 [email protected] Chief Editor Samachar 5067200, 201 Group 2212121 2212124 2280104-07 F. 2280111-14 02. Avinash Chopra Hind 2280104-07 98140-64964 [email protected] Joint Editor Samachar F. 2401022 2280104-09 Group 03. S. Barjinder Ajit Group 2455961,62, [email protected] Singh Hamdard 63,2458588 [email protected] Managing Editor 5032400 F. 2220593 2455960 04. Jagjit Singh Nawan 2220290 98150-36297 [email protected] Anand Zamana 2230466 2271303 Chief Editor 2235771 F. 2240280 F.2222409 05. Gurdeep Singh Aj Di Awaj 4626370,71 98760-72000 [email protected] Chief Editor 2234700 [email protected] F. 2235700 F. 4626369 06. Birpal Singh Akali Patrika 5008586 98721-00035 [email protected] Chief Editor F . 2284694 [email protected] 07. Minakshi Sharma Dainik 3941300 98729-00202 [email protected] Editor Jagran 3067500 F. 3067555 F. 2600267 08. Nishikant Thakur Dainik 3941300 98100-53283 [email protected] Resident Editor Jagran 3067500 F. 3067555 F. 2600267 09. S.S.Bhullar Punjabi ----do--- 81466-80402 [email protected] Editor Jagran 1 10. Yogeshwar Dutt Dainik 3988884 99881-35004 [email protected] Syal Bhaskar 2222016 Resident Editor F. 3985311 11. Irwin Khanna Uttam Hindu 2602838 94172-27722 [email protected] Chief Editor 2601557 2458707 [email protected] 2602076 F. 5058823 F. 2602762 12. Chander Mohan Vir Partap 2457867,68 98154-30709 [email protected] Chief Editor F. -

Journal of Media Studies Vol
Journal of Media Studies Vol. 28(2): July 2013 146-160 © 2010 ICS Publications www.jms.edu.pk Male political photographs dominate on front pages: A content analysis of front page political visuals of leading Indian Newspapers Pradeep Kumar Tewari1 Abstract This study examines the gender representation of political photographs from the most circulated newspapers in India. A total of 355 political photographs were yielded from the 336 front pages among the selected newspapers. Important finding of the research are male political photographs (72%) dominate in the Indian newspapers. Dainik Tribune published the maximum photographs of male politicians among the selected newspapers. Maximum political photographs of men were used with the caption. Most of the women political photographs were used with the story. Percentage of international photographs of women politicians was higher than male politicians. English newspapers published maximum women political photographs as compared to vernacular newspapers. All the selected newspapers have given only 3% space to all the women politicians. Keywords: Photojournalism, Gender study, Content analysis, Front page, Political photographs. Introduction Photographs are an important part of news reporting since the beginning of photography. Before the advent of photography, newspapers were using illustrations to explain the news events. According to famous art historian Gombrich (1996), "We are living in a visual age. We are bombarded by pictures from morning to night. Opening our newspaper at breakfast we see photographs of men and women in the news, and raising our eyes from the paper, we encounter the picture on the cereal package". During the political news coverage, photographs play an important role to show the reality of political circles. -

A Case Study of Coverage of Human Right Issues in Selected Kannada Language Newspapers
© 2019 JETIR January 2019, Volume 6, Issue 1 www.jetir.org (ISSN-2349-5162) MEDIA AS HUMAN RIGHT EDUCATOR: A CASE STUDY OF COVERAGE OF HUMAN RIGHT ISSUES IN SELECTED KANNADA LANGUAGE NEWSPAPERS 1Dr.Moulya Jeevanram, Associate Professor, Department of PG studies in Journalism, Alva’s College, Moodubidire 2Shrigouri S. Joshi, student, Department of PG studies in Journalism, Alva’s College 3Dr Kushal Kumar School of Humanities Lovely Professional University Punjab. Abstract Human rights are moral principles or norms which describe certain standards of human behavior, and are regularly protected as legal rights in municipal and international law. There are many substantive rights of human rights such as right to life, freedom from torture, freedom from slavery, right to a fair trial etc; but unfortunately most of the time people don’t know them. People should be aware of Human rights. Educating society on human rights is an essential task. Media is a field which is doing this job very efficiently since long time. Media is like a mirror. Its main task is to gather the information and reflect it back in proper form. Media are the producers of information for general public. This information includes human rights issues most of the times. Human rights are compass for good journalism. Journalists have a moral obligation to promote human rights all the time and in every story. Media are like watch dogs, who warn the people about threats to their freedom, to their society, to their livelihood and to their culture. The present research paper attempted to observe the coverage of human rights in five major Kannada newspapers of one month and also attempted to find out how people get educated through the news published about Human Rights. -

Recognized Outlets for Svran Apeejay Journalism Foundation Grant
Recognized Outlets for Svran Apeejay Journalism Foundation Grant PRINT PUBLICATIONS Nai Duniya Nav Bharat Times (Hindi) Aajkal Nav Gujarat Samay Ahmedabad Mirror Navodaya Times (Hindi) Ajit Samachar(Hindi) Orissa Post Ajit(Punjabi) Pioneer (Hindi) Amar Ujala Punjab Kesari (Hindi), Editions: Punjab, Anand Bazar Patrika(Bengali) Haryana, Rajasthan, Himachal Pradesh, Bangalore Mirror Delhi, UP Bartaman (Bengali) Prabhat Khabar Business Standard Prajavani Daily Ajit(Urdu) Rajasthan Patrika Dainik Bhaskar Sakaal Times Dainik Divya Marathi(Marathi) Sanjevani Dainik Jagran Sangbad Pratidin Dainik Statesman (Bengali) Samyukta Karnataka Dainik Tribune (Hindi) The Sentinel DB Post (English) The Shillong Times Dinamalar The Hans India Dinamani The Asian Age Dinakaran The Deccan Chronicle Divya Bhaskar (Gujrat) The Deccan Herald DNA (English) The Financial Express Ebela (Bengali Tabloid) The Financial Chronicle Economic Times The Hindu Ei Samay The Hindu Business Line Ganashakti The Hindu (Tamil) Gujarat Samachar The New Indian Express Hind Samachar (Urdu) The Pioneer Hindustan Times The Statesman Hindustan (Hindi) The Telegraph Indian Express The Tribune Jag Bani (Punjabi) Times of India Jansatta Uttarbanga Sangbad Kesari Vijaya Karnataka (Kannadi) Lokmat (Marathi), Editions: Mumbai, The Assam Tribune Pune, Nagpur, Aurangabad, Nashik, Malyala Manorama Kolhapur, Sangli, Satara, Solapur, Akola, Ahmed Nagar, Jalgaon, Goa Loksatta (Marathi) MAGAZINES Maharashtra Times (Marathi) Mail Today (Tabloid) BBC Knowledge Mid-Day (Tabloid available in the Business