The Desmosome and Pemphigus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Biologic Agents in the Treatment of Oral Lesions Due to Pemphigus and Behçet's Disease: a Systematic Review
Davis GE, Sarandev G, Vaughan AT, Al-Eryani K, Enciso R. The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behçet’s Disease: A Systematic Review. J Anesthesiol & Pain Therapy. 2020;1(1):14-23 Systematic Review Open Access The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behçet’s Disease: A Systematic Review Gerald E. Davis II1,2, George Sarandev1, Alexander T. Vaughan1, Kamal Al-Eryani3, Reyes Enciso4* 1Advanced graduate, Master of Science Program in Orofacial Pain and Oral Medicine, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA 2Assistant Dean of Academic Affairs, Assistant Professor, Restorative Dentistry, Meharry Medical College, School of Dentistry, Nashville, Tennessee, USA 3Assistant Professor of Clinical Dentistry, Division of Periodontology, Dental Hygiene & Diagnostic Sciences, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA 4Associate Professor (Instructional), Division of Dental Public Health and Pediatric Dentistry, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA Article Info Abstract Article Notes Background: Current treatments for pemphigus and Behçet’s disease, such Received: : March 11, 2019 as corticosteroids, have long-term serious adverse effects. Accepted: : April 29, 2020 Objective: The objective of this systematic review was to evaluate the *Correspondence: efficacy of biologic agents (biopharmaceuticals manufactured via a biological *Dr. Reyes Enciso, Associate Professor (Instructional), Division source) on the treatment of intraoral lesions associated with pemphigus and of Dental Public Health and Pediatric Dentistry, Herman Ostrow Behçet’s disease compared to glucocorticoids or placebo. School of Dentistry of USC, Los Angeles, California, USA; Email: [email protected]. -

Medicare Human Services (DHHS) Centers for Medicare & Coverage Issues Manual Medicaid Services (CMS) Transmittal 155 Date: MAY 1, 2002
Department of Health & Medicare Human Services (DHHS) Centers for Medicare & Coverage Issues Manual Medicaid Services (CMS) Transmittal 155 Date: MAY 1, 2002 CHANGE REQUEST 2149 HEADER SECTION NUMBERS PAGES TO INSERT PAGES TO DELETE Table of Contents 2 1 45-30 - 45-31 2 2 NEW/REVISED MATERIAL--EFFECTIVE DATE: October 1, 2002 IMPLEMENTATION DATE: October 1, 2002 Section 45-31, Intravenous Immune Globulin’s (IVIg) for the Treatment of Autoimmune Mucocutaneous Blistering Diseases, is added to provide limited coverage for the use of IVIg for the treatment of biopsy-proven (1) Pemphigus Vulgaris, (2) Pemphigus Foliaceus, (3) Bullous Pemphigoid, (4) Mucous Membrane Pemphigoid (a.k.a., Cicatricial Pemphigoid), and (5) Epidermolysis Bullosa Acquisita. Use J1563 to bill for IVIg for the treatment of biopsy-proven (1) Pemphigus Vulgaris, (2) Pemphigus Foliaceus, (3) Bullous Pemphigoid, (4) Mucous Membrane Pemphigoid, and (5) Epidermolysis Bullosa Acquisita. This revision to the Coverage Issues Manual is a national coverage decision (NCD). The NCDs are binding on all Medicare carriers, intermediaries, peer review organizations, health maintenance organizations, competitive medical plans, and health care prepayment plans. Under 42 CFR 422.256(b), an NCD that expands coverage is also binding on a Medicare+Choice Organization. In addition, an administrative law judge may not review an NCD. (See §1869(f)(1)(A)(i) of the Social Security Act.) These instructions should be implemented within your current operating budget. DISCLAIMER: The revision date and transmittal number only apply to the redlined material. All other material was previously published in the manual and is only being reprinted. CMS-Pub. -

Pemphigus. S2 Guideline for Diagnosis and Treatment
DOI: 10.1111/jdv.12772 JEADV GUIDELINES Pemphigus. S2 Guideline for diagnosis and treatment – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) M. Hertl,1,* H. Jedlickova,2 S. Karpati,3 B. Marinovic,4 S. Uzun,5 S. Yayli,6 D. Mimouni,7 L. Borradori,8 C. Feliciani,9 D. Ioannides,10 P. Joly,11 C. Kowalewski,12 G. Zambruno,13 D. Zillikens,14 M.F. Jonkman15 1Department of Dermatology, Philipps-University Marburg, Marburg, Germany 2Department of Dermatology, Masaryk University, Brno, Czech Republic 3Department of Dermatology, Semmelweis University Budapest, Budapest, Hungary 4Department of Dermatology, School of Medicine University of Zagreb, Zagreb, Croatia 5Department of Dermatology, Akdeniz University, Antalya, Turkey 6Department of Dermatology, Karadeniz Technical University, Trabzon, Turkey 7Department of Dermatology, Tel-Aviv University, Tel-Aviv, Israel 8Department of Dermatology, University of Bern, Inselspital, Switzerland 9Department of Dermatology, University of Parma, Parma, Italy 10Department of Dermatology, Aristotle University of Thessaloniki, Thessaloniki, Greece 11Department of Dermatology, Rouen University Hospital, Rouen, France 12Department of Dermatology, Medical University of Warsaw, Warsaw, Poland 13Department of Dermatology, L’Istituto Dermopatico dell’Immacolata, Rome, Italy 14Department of Dermatology, University of Lubeck,€ Lubeck,€ Germany 15Department of Dermatology, University of Groningen, Groningen, The Netherlands *Correspondence: M. Hertl. E-mail: [email protected] Abstract Background Pemphigus encompasses a group of life-threatening autoimmune bullous diseases characterized by blis- ters and erosions of the mucous membranes and skin. Before the era of immunosuppressive treatment, the prognosis of pemphigus was almost fatal. Due to its rarity, only few prospective controlled therapeutic trials are available. -

Pemphigus Foliaceus Igg Causes Dissociation of Desmoglein 1– Containing Junctions Without Blocking Desmoglein 1 Transinteraction
Pemphigus foliaceus IgG causes dissociation of desmoglein 1– containing junctions without blocking desmoglein 1 transinteraction Jens Waschke, … , Detlef Zillikens, Detlev Drenckhahn J Clin Invest. 2005;115(11):3157-3165. https://doi.org/10.1172/JCI23475. Research Article Dermatology Autoantibodies against the epidermal desmosomal cadherins desmoglein 1 (Dsg1) and Dsg3 have been shown to cause severe to lethal skin blistering clinically defined as pemphigus foliaceus (PF) and pemphigus vulgaris (PV). It is unknown whether antibody-induced dissociation of keratinocytes is caused by direct inhibition of Dsg1 transinteraction or by secondary cellular responses. Here we show in an in vitro system that IgGs purified from PF patient sera caused cellular dissociation of cultured human keratinocytes as well as significant release of Dsg1-coated microbeads attached to Dsg- containing sites on the keratinocyte cellular surface. However, cell dissociation and bead release induced by PF-IgGs was not caused by direct steric hindrance of Dsg1 transinteraction, as demonstrated by single molecule atomic force measurements and by laser trapping of surface-bound Dsg1-coated microbeads. Rather, our experiments strongly indicate that PF-IgG–mediated dissociation events must involve autoantibody-triggered cellular signaling pathways, resulting in destabilization of Dsg1-based adhesive sites and desmosomes. Find the latest version: https://jci.me/23475/pdf Research article Pemphigus foliaceus IgG causes dissociation of desmoglein 1–containing junctions without blocking desmoglein 1 transinteraction Jens Waschke,1 Paola Bruggeman,1 Werner Baumgartner,1 Detlef Zillikens,2 and Detlev Drenckhahn1 1Institute of Anatomy and Cell Biology, University of Würzburg, Würzburg, Germany. 2Department of Dermatology, University of Lübeck, Lübeck, Germany. Autoantibodies against the epidermal desmosomal cadherins desmoglein 1 (Dsg1) and Dsg3 have been shown to cause severe to lethal skin blistering clinically defined as pemphigus foliaceus (PF) and pemphigus vulgaris (PV). -
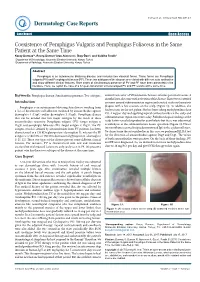
Coexistence of Pemphigus Vulgaris and Pemphigus Foliaceus in the Same Patient at the Same Time
logy Case to R a ep rm o e r Durmaz et al., Dermatol Case Rep 2017,2:1 t D Dermatology Case Reports ResearchCase Report Article Open Access Coexistence of Pemphigus Vulgaris and Pemphigus Foliaceus in the Same Patient at the Same Time Koray Durmaz1*, Recep Dursun1 Arzu Ataseven1, İlkay Özer1, and Siddika Fındik2 1Department of Dermatology, Necmettin Erbakan University, Konya, Turkey 2Department of Pathology, Necmettin Erbakan University, Konya, Turkey Abstract Pemphigus is an autoimmune blistering disease and includes two classical forms. These forms are Pemphigus vulgaris (PV) and Pemphigus foliaceus (PF). These two subtypes of the disease are related with different auto-antibodies and show different clinical features. Rare cases of simultaneous presence of PV and PF have been presented in the literature. Here, we report the case of a 62-year-old woman who developed PV and PF lesions at the same time. Keywords: Pemphigus disease; Simultaneous presence; Two subtypes control visits after 5 IVIG infusions because of some personal reasons. 8 months later, she came with activation of the disease. There were scattered Introduction erosions around submammarian region and crusted scaly erythematous Pemphigus is an autoimmune blistering skin disease resulting from plaques with a few erosions on the scalp (Figure 1). In addition, she a loss of keratinocyte cell adhesion mediated by autoantibodies against had erosions on the soft palate. She has been taking methylprednisolone desmoglein 1 (Dsg1) and/or desmoglein 3 (Dsg3). Pemphigus disease P.O. 4 mg per day and applying topical corticosteroids on the scalp and that can be divided into two major subtypes by the result of these submammarian region two times a day. -

Invited Review Desmosomes and Disease
Histol Histopathol (1997) 12: 1159-1168 Histology and 001: 10.14670/HH-12.1159 Histopathology http://www.hh.um.es From Cell Biology to Tissue Engineering Invited Review Desmosomes and disease M.A.J. Chidgey School of Biological Sciences, University of Manchester, Manchester, UK Summary. Considerable progress has been made in our and dendritic reticulum cells of lymphatic follicles. They knowledge of desmosomes and their components. are less than I !lm in diameter and show characteristic Molecular cloning of the desmosomal glycoproteins has features when viewed by electron microscopy (Fig. I A). established that desmoglein I and desmoglein 3 are In cross section desmosomes generally appear as targets for autoantibodies in the blistering diseases electron dense discs at sites of close cell-cell contact. pemphigus foliaceus and pemphigus vulgaris The plasma membranes of each cell are separated by respectively. New evidence suggests that another dense material (the midline) with lateral projections desmosomal glycoprotein, desmocollin I, is the major radiating to the membrane. The intracellular material is target antigen in the upper epidermal form of highly organised and consists of an electron dense intercellular IgA dermatosis (lgA pemphigus). In human plaque that can vary in appearance from one cell type to cancer there is accumulating evidence which suggests a another. Intermediate filaments (lFs) of the cytoskeleton role for desmosomes in the prevention of invasion and anchor at the plaque. In epithelia desmosomes associate metastasis. The possibility exists that a mutation in a with keratin-containing IFs but they are also able to desmosomal glycoprotein gene is responsible for an interact with IFs containing either desmin or vimentin. -

Radiation-Induced Pemphigus Or Pemphigoid Disease in 3 Patients with Distinct Underlying Malignancies
Radiation-Induced Pemphigus or Pemphigoid Disease in 3 Patients With Distinct Underlying Malignancies Wonwoo Shon, DO; David A. Wada, MD; Amer N. Kalaaji, MD PRACTICE POINTS • The use of radiation therapy is increasing because of its therapeutic benefit, especially in advanced-stage cancer patients. • Although there is a wide range of adverse effects associated with radiation therapy, pemphigus or pemphigoid disease is rare and needs to be distinguished from other skin diseases or even recurrent underlying cancer. • The precise mechanism of radiation-induced pemphigus or pemphigoid disease is unknown, but clinicians should be alert to this potentially serious complication, and all cutaneous eruptions developing during and after radiation therapy should be evaluated with routine histologic examination in conjunction with direct immunofluorescence, serum for indirect immunofluorescence, and enzyme-linked immunosorbent assay. The cutaneous lesions of radiation-induced pem- eruption is unknown, clinicians should be alert for phigus or pemphigoid disease may resemble this potentially serious complication and evaluate other skin diseases, including recurrent underly- all cutaneous eruptions developing during and ing cancer. We performed a computerized search after radiotherapy. of Mayo Clinic (Rochester, Minnesota) archives Cutis. 2016;97:219-222. and identified 3 cases of pemphigus or pemphi- goid disease that occurredCUTIS after radiation therapy Do not copy for breast, cervical, and metastatic malignancies, number of adverse cutaneous effects may respectively. In 2 of these patients, the disease result from radiation therapy, including was initially confined to the irradiated field but A radiodermatitis, alopecia, and radiation- subsequently disseminated to other parts of the induced neoplasms. Radiation therapy rarely induces patients’ bodies, including mucosal surfaces. -

SKIN VERSUS PEMPHIGUS FOLIACEUS and the AUTOIMMUNE GANG Lara Luke, BS, RVT, Dermatology, Purdue Veterinary Teaching Hospital
VETERINARY NURSING EDUCATION SKIN VERSUS PEMPHIGUS FOLIACEUS AND THE AUTOIMMUNE GANG Lara Luke, BS, RVT, Dermatology, Purdue Veterinary Teaching Hospital This program was reviewed and approved by the AAVSB Learning Objective: After reading this article, the participant will be able to dis- RACE program for 1 hour of continuing education in jurisdictions which recognize AAVSB RACE approval. cuss and compare autoimmune diseases that have dermatological afects, includ- Please contact the AAVSB RACE program if you have any ing Pemphigus Foliaceus (PF), Pemphigus Erythematosus (PE), Discoid Lupus comments/concerns regarding this program’s validity or relevancy to the veterinary profession. Erythematosus (DLE), Systemic Lupus Erythematosus (SLE). In addition, the reader will become familiar with diagnostic and treatment techniques. FUNCTION OF THE SKIN Te skin is the largest organ of the body. Along with sensory function, it provides a barrier between the inside and outside world. Te epidermis is composed of the following fve layers: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. Te stratum lucidum is found only on the nasal planum and footpads. When the cells of the epidermis are disrupted by systemic disease, the barrier is also disrupted. Clinical signs of skin disease will bring the patient into the veterinarian’s ofce for diagnosis. 32 THE NAVTA JOURNAL | NAVTA.NET VETERINARY NURSING EDUCATION THE PEMPHIGUS COMPLEX article.3 Histologically it shares characteris- Pemphigus Foliaceus tics of both PF and DLE.1 This classifcation PF is an immune mediated pustular disor- is still considered controversial and PE may der included in a group of diseases known just be a localized variant of PF.1 as the pemphigus complex. -

Think Clinical Immunodermatology Laboratory
Clinical ImmunodermatologyThink ImmunodermatologyLaboratory Testing! Kristin M. Leiferman, M.D. Professor of Dermatology Co-Director Immunodermatology Laboratory University of Utah History Late 1800s Paul Ehrlich put forth the concept of autoimmunity calling it “horror autotoxicus” History Early 1940s Albert Coons was the first to conceptualize and develop immunofluorescent techniques for labeling antibodies History 1945 Robin Coombs (and colleagues) described the Coombs antiglobulin reaction test, used to determine if antibodies or complement factors have bound to red blood cell surface antigens in vivo causing hemolytic anemia •Waaler-Rose rheumatoid factor •Hargraves’ LE cell •Witebsky-Rose induction of thyroiditis with autologous thyroid gland History Mid 1960s Ernest Beutner and Robert Jordon demonstrated IgG cell surface antibodies in pemphigus, autoantibodies in circulation and bound to the dermal-epidermal junction in bullous pemphigoid Immunobullous Diseases Immunobullous Diseases • Desmogleins / Desmosomes – Pemphigus • BP Ags in hemidesmosomes / lamina lucida – Pemphigoid – Linear IgA bullous dermatosis • Type VII collagen / anchoring fibrils – Epidermolysis bullosa acquisita Immunodermatology Tests are Diagnostic Aids in Many Diseases • Dermatitis herpetiformis & • Mixed / undefined celiac disease connective tissue disease • Drug reactions • Pemphigoid (all types) • Eosinophil-associated disease • Pemphigus (all types, including paraneoplastic) • Epidermolysis bullosa acquisita • Porphyria & pseudoporphyria • Lichen planus -

Feline Pemphigus Foliaceus
SERIES EDITOR Craig E. Griffin, DVM, DACVD Animal Dermatology Clinic, San Diego, California SERIES EDITOR Wayne S. Rosenkrantz, DVM, DACVD Animal Dermatology Clinic, Tustin, California Crusty Cats: Feline Pemphigus Foliaceus Andrea Peterson, DVM University of Minnesota Lindsay McKay, DVM, DACVD* VCA Aurora Animal Hospital Aurora, Illinois Abstract: Pemphigus foliaceus (PF) is an immune-mediated disease that causes pustules and crusted lesions, most commonly on the pinnae, nasal planum, periocular area, chin, and feet of affected cats. Acantholytic cells caused by degradation of intercellular adhesions are often seen on cytology but are not pathognomonic for PF. A definitive diagnosis is made based on histopathology showing subcorneal pustules with nondegenerate neutrophils and acantholytic cells. PF is treated with immunosuppressive doses of corticosteroids alone or in combination with other immunosuppressive medications, such as chlorambucil or cyclosporine. Most patients require lifelong treatment with these medications to keep the disease in remission. Case Report dition score of 4/9, and was clinically mildly dehydrated. Hershey, a 6-year-old, spayed domestic shorthaired cat Other general examination findings were normal. Crusts, weighing 3.4 kg, presented with an acute onset of nonpru- erosions, and scaling were present on both pinnae (FIGURE ritic crusted lesions on the head, ears, nail beds, and nasal 1). Her chin had alopecia, erythema, and crusts. Additional area. She had a 2-day history of lethargy and anorexia. She crusts were found around her eyes, on the top of her head, had no history of medical disease and was up-to-date on on her muzzle, and in her nail beds. The nasal planum vaccinations. -

Blistering Skin Conditions
THEME WEIRD SKIN STUFF Belinda Welsh MBBS, MMed, FACD, is consultant dermatologist, St Vincent's Hospital, Melbourne and Sunbury Dermatology and Skin Cancer Clinic, Sunbury, Victoria. [email protected] Blistering skin conditions Blistering of the skin is a reaction pattern to a diverse Background group of aetiologic triggers and can be classified as either: Blistering of the skin can be due to a number of diverse • immunobullous (Table 1), or aetiologies. Pattern and distribution of blisters can be helpful in • nonimmunobullous (Table 2). diagnosis but usually biopsy is required for histopathology and immunofluoresence to make an accurate diagnosis. Separation of the skin layers leading to acquired blistering can occur due to loss of cohesion of cells: Objective • within the epidermis (Figure 1) This article outlines the clinical and pathological features of • between the epidermis and dermis (basement membrane blistering skin conditions with a particular focus on bullous zone) (Figure 2), or impetigo, dermatitis herpetiformis, bullous pemphigoid and • in the uppermost layers of the dermis. porphyria cutanea tarda. Discussion This distinction forms the histologic basis of diagnosing many of the Infections, contact reactions and drug eruptions should different blistering diseases. Clinical patterns may also be helpful and always be considered. Occasionally blistering may represent are listed in Table 3. Important features include: a cutaneous manifestation of a metabolic disease such as • location of the blisters (Figure 3, 4) porphyria. Although rare, it is important to be aware of the autoimmune group of blistering diseases, as if unrecognised and • the presence or absence of mucosal involvement, and untreated, they can lead to significant morbidity and mortality. -

A Review of Acquired Autoimmune Blistering Diseases in Inherited Epidermolysis Bullosa: Implications for the Future of Gene Therapy
antibodies Review A Review of Acquired Autoimmune Blistering Diseases in Inherited Epidermolysis Bullosa: Implications for the Future of Gene Therapy Payal M. Patel 1,2 , Virginia A. Jones 1 , Christy T. Behnam 1, Giovanni Di Zenzo 3 and Kyle T. Amber 2,4,* 1 Department of Dermatology, University of Illinois at Chicago College of Medicine, Chicago, IL 60612, USA; [email protected] (P.M.P.); [email protected] (V.A.J.); [email protected] (C.T.B.) 2 Rush University Medical Center, Division of Dermatology, Department of Otorhinolaryngology—Head and Neck Surgery, Rush University, Chicago, IL 60612, USA 3 Laboratory of Molecular and Cell Biology, IDI-IRCCS, 00167 Rome, Italy; [email protected] 4 Rush University Medical Center, Department of Internal Medicine, Rush University, Chicago, IL 60612, USA * Correspondence: [email protected] Abstract: Gene therapy serves as a promising therapy in the pipeline for treatment of epidermolysis bullosa (EB). However, with great promise, the risk of autoimmunity must be considered. While EB is a group of inherited blistering disorders caused by mutations in various skin proteins, autoimmune blistering diseases (AIBD) have a similar clinical phenotype and are caused by autoantibodies targeting skin antigens. Often, AIBD and EB have the same protein targeted through antibody or mutation, respectively. Moreover, EB patients are also reported to carry anti-skin antibodies of questionable pathogenicity. It has been speculated that activation of autoimmunity is both a consequence and cause of further skin deterioration in EB due to a state of chronic inflammation. Citation: Patel, P.M.; Jones, V.A.; Behnam, C.T.; Zenzo, G.D.; Amber, Herein, we review the factors that facilitate the initiation of autoimmune and inflammatory responses K.T.