A Tale on the Demersal and Bottom Dwelling Chondrichthyes in the South of Sicily
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fisheries Centre
Fisheries Centre The University of British Columbia Working Paper Series Working Paper #2015 - 80 Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulman, Adib Saad, Kyrstn Zylich, Daniel Pauly and Dirk Zeller Year: 2015 Email: [email protected] This working paper is made available by the Fisheries Centre, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada. Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulmana, Adib Saadb, Kyrstn Zylicha, Daniel Paulya, Dirk Zellera a Sea Around Us, Fisheries Centre, University of British Columbia, 2202 Main Mall, Vancouver, BC, V6T 1Z4, Canada b President of Syrian National Committee for Oceanography, Tishreen University, Faculty of Agriculture, P.O. BOX; 1408, Lattakia, Syria [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected] ABSTRACT Syria’s total marine fisheries catches were estimated for the 1950-2010 time period using a reconstruction approach which accounted for all fisheries removals, including unreported commercial landings, discards, and recreational and subsistence catches. All unreported estimates were added to the official data, as reported by the Syrian Arab Republic to the United Nation’s Food and Agriculture Organization (FAO). Total reconstructed catch for 1950-2010 was around 170,000 t, which is 78% more than the amount reported by Syria to the FAO as their national catch. The unreported components added over 74,000 t of unreported catches, of which 38,600 t were artisanal landings, 16,000 t industrial landings, over 4,000 t recreational catches, 3,000 t subsistence catches and around 12,000 t were discards. -

Shark Cartilage, Cancer and the Growing Threat of Pseudoscience
[CANCER RESEARCH 64, 8485–8491, December 1, 2004] Review Shark Cartilage, Cancer and the Growing Threat of Pseudoscience Gary K. Ostrander,1 Keith C. Cheng,2 Jeffrey C. Wolf,3 and Marilyn J. Wolfe3 1Department of Biology and Department of Comparative Medicine, Johns Hopkins University, Baltimore, Maryland; 2Jake Gittlen Cancer Research Institute, Penn State College of Medicine, Hershey, Pennsylvania; and 3Registry of Tumors in Lower Animals, Experimental Pathology Laboratories, Inc., Sterling, Virginia Abstract primary justification for using crude shark cartilage extracts to treat cancer is based on the misconception that sharks do not, or infre- The promotion of crude shark cartilage extracts as a cure for cancer quently, develop cancer. Other justifications represent overextensions has contributed to at least two significant negative outcomes: a dramatic of experimental observations: concentrated extracts of cartilage can decline in shark populations and a diversion of patients from effective cancer treatments. An alleged lack of cancer in sharks constitutes a key inhibit tumor vessel formation and tumor invasions (e.g., refs. 2–5). justification for its use. Herein, both malignant and benign neoplasms of No available data or arguments support the medicinal use of crude sharks and their relatives are described, including previously unreported shark extracts to treat cancer (6). cases from the Registry of Tumors in Lower Animals, and two sharks with The claims that sharks do not, or rarely, get cancer was originally two cancers each. Additional justifications for using shark cartilage are argued by I. William Lane in a book entitled “Sharks Don’t Get illogical extensions of the finding of antiangiogenic and anti-invasive Cancer” in 1992 (7), publicized in “60 Minutes” television segments substances in cartilage. -

Sharks in Crisis: a Call to Action for the Mediterranean
REPORT 2019 SHARKS IN CRISIS: A CALL TO ACTION FOR THE MEDITERRANEAN WWF Sharks in the Mediterranean 2019 | 1 fp SECTION 1 ACKNOWLEDGEMENTS Written and edited by WWF Mediterranean Marine Initiative / Evan Jeffries (www.swim2birds.co.uk), based on data contained in: Bartolí, A., Polti, S., Niedermüller, S.K. & García, R. 2018. Sharks in the Mediterranean: A review of the literature on the current state of scientific knowledge, conservation measures and management policies and instruments. Design by Catherine Perry (www.swim2birds.co.uk) Front cover photo: Blue shark (Prionace glauca) © Joost van Uffelen / WWF References and sources are available online at www.wwfmmi.org Published in July 2019 by WWF – World Wide Fund For Nature Any reproduction in full or in part must mention the title and credit the WWF Mediterranean Marine Initiative as the copyright owner. © Text 2019 WWF. All rights reserved. Our thanks go to the following people for their invaluable comments and contributions to this report: Fabrizio Serena, Monica Barone, Adi Barash (M.E.C.O.), Ioannis Giovos (iSea), Pamela Mason (SharkLab Malta), Ali Hood (Sharktrust), Matthieu Lapinksi (AILERONS association), Sandrine Polti, Alex Bartoli, Raul Garcia, Alessandro Buzzi, Giulia Prato, Jose Luis Garcia Varas, Ayse Oruc, Danijel Kanski, Antigoni Foutsi, Théa Jacob, Sofiane Mahjoub, Sarah Fagnani, Heike Zidowitz, Philipp Kanstinger, Andy Cornish and Marco Costantini. Special acknowledgements go to WWF-Spain for funding this report. KEY CONTACTS Giuseppe Di Carlo Director WWF Mediterranean Marine Initiative Email: [email protected] Simone Niedermueller Mediterranean Shark expert Email: [email protected] Stefania Campogianni Communications manager WWF Mediterranean Marine Initiative Email: [email protected] WWF is one of the world’s largest and most respected independent conservation organizations, with more than 5 million supporters and a global network active in over 100 countries. -

Extinction Risk and Conservation of the World's Sharks and Rays
RESEARCH ARTICLE elife.elifesciences.org Extinction risk and conservation of the world’s sharks and rays Nicholas K Dulvy1,2*, Sarah L Fowler3, John A Musick4, Rachel D Cavanagh5, Peter M Kyne6, Lucy R Harrison1,2, John K Carlson7, Lindsay NK Davidson1,2, Sonja V Fordham8, Malcolm P Francis9, Caroline M Pollock10, Colin A Simpfendorfer11,12, George H Burgess13, Kent E Carpenter14,15, Leonard JV Compagno16, David A Ebert17, Claudine Gibson3, Michelle R Heupel18, Suzanne R Livingstone19, Jonnell C Sanciangco14,15, John D Stevens20, Sarah Valenti3, William T White20 1IUCN Species Survival Commission Shark Specialist Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 2Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 3IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, Newbury, United Kingdom; 4Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, United States; 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom; 6Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Australia; 7Southeast Fisheries Science Center, NOAA/National Marine Fisheries Service, Panama City, United States; 8Shark Advocates International, The Ocean Foundation, Washington, DC, United States; 9National Institute of Water and Atmospheric Research, Wellington, New Zealand; 10Global Species Programme, International Union for the Conservation -

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

Studies of a Goneplax Rhomboides Population Off Quarry Bay 55
Studies of a Goneplax rhomboides population off Quarry Bay R. J. A. ATKINSON Dept of Marine Biology, University of Liverpool, Isle of Man Present address: University Marine Biological Station, Millport, Isle of Cumbrae, Scotland. Introduction This work was conducted during a course in sublittoral marine ecology sponsored by the Lundy Field Society and the Department of Marine Biology, University College of North Wa les, Menai Bridge, Anglesey. The observations reported here result from a team effort in volving Mr. A.R. Andrews, Miss M. Cooper, Mr. J. Creak, Mr. M. Davies, Dr. P.M. Hardy , Mr. J.B . Markham , Mr . C. Roberts, Mr. D. Tierney, Mr. J.G. Wilson and myself. The main objects of the exercise were to familiarise ourselves with various types of burows in the mud and employ a number of techniques to study them. This involved the preparation of polyester resin casts of selected burrows, mapping the distribution of burrows and observing the area by night as well as by day. Although Goneplax rhomboides (L.) was responsible for must of the conspicuous burrows in the area several distinctive burrows occupied by the Red Band Fish (Cepola rnbescens L.) were fou nd .. The squat lobster Munida bamffica (Pennant) was occasionally seen in th e en trances of burrows but these burrows appeared to be vacated Goneplax burrows. Methods A 48 metre tape measure was pinned into the mud substratum 17m below C.D. off Quarry Bay. Those burrows that occurred within lm either side of the tape were mapped using pencils on prepared perspex 'slates'. Interrelationships between holes in th e mud we re ascertained by creating a current of water in the burrow and noting those openings through which mud clouds emerged. -

Spatial Ecology and Fisheries Interactions of Rajidae in the Uk
UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences SPATIAL ECOLOGY AND FISHERIES INTERACTIONS OF RAJIDAE IN THE UK Samantha Jane Simpson Thesis for the degree of DOCTOR OF PHILOSOPHY APRIL 2018 UNIVERSITY OF SOUTHAMPTON 1 2 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Sciences Doctor of Philosophy FINE-SCALE SPATIAL ECOLOGY AND FISHERIES INTERACTIONS OF RAJIDAE IN UK WATERS by Samantha Jane Simpson The spatial occurrence of a species is a fundamental part of its ecology, playing a role in shaping the evolution of its life history, driving population level processes and species interactions. Within this spatial occurrence, species may show a tendency to occupy areas with particular abiotic or biotic factors, known as a habitat association. In addition some species have the capacity to select preferred habitat at a particular time and, when species are sympatric, resource partitioning can allow their coexistence and reduce competition among them. The Rajidae (skate) are cryptic benthic mesopredators, which bury in the sediment for extended periods of time with some species inhabiting turbid coastal waters in higher latitudes. Consequently, identifying skate fine-scale spatial ecology is challenging and has lacked detailed study, despite them being commercially important species in the UK, as well as being at risk of population decline due to overfishing. This research aimed to examine the fine-scale spatial occurrence, habitat selection and resource partitioning among the four skates across a coastal area off Plymouth, UK, in the western English Channel. In addition, I investigated the interaction of Rajidae with commercial fisheries to determine if interactions between species were different and whether existing management measures are effective. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Batoid Abundances, Spatial Distribution, and Life History Traits
animals Article Batoid Abundances, Spatial Distribution, and Life History Traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a Knowledge Gap through Three Decades of Survey Michele Luca Geraci 1,2 , Sergio Ragonese 2,*, Danilo Scannella 2, Fabio Falsone 2, Vita Gancitano 2 , Jurgen Mifsud 3, Miriam Gambin 3, Alicia Said 3 and Sergio Vitale 2 1 Geological and Environmental Sciences (BiGeA)–Marine Biology and Fisheries Laboratory, Department of Biological, University of Bologna, Viale Adriatico 1/n, 61032 Fano, PU, Italy; [email protected] 2 Institute for Marine Biological Resources and Biotechnology (IRBIM), National Research Council–CNR, Via Luigi Vaccara, 61, 91026 Mazara del Vallo, TP, Italy; [email protected] (D.S.); [email protected] (F.F.); [email protected] (V.G.); [email protected] (S.V.) 3 Department of Fisheries and Aquaculture, Ministry for Agriculture, Fisheries and Animal Rights (MAFA), Ghammieri Government Farm, Triq l-Ingiered, Malta; [email protected] (J.M.); [email protected] (M.G.); [email protected] (A.S.) * Correspondence: [email protected] Simple Summary: Batoid species are cartilaginous fish commonly known as rays, but they also Citation: Geraci, M.L.; Ragonese, S.; include stingrays, electric rays, guitarfish, skates, and sawfish. These species are very sensitive Scannella, D.; Falsone, F.; Gancitano, to fishing, mainly because of their slow growth rate and late maturity; therefore, they need to be V.; Mifsud, J.; Gambin, M.; Said, A.; adequately managed. Regrettably, information on life history traits (e.g., length at first maturity, Vitale, S. Batoid Abundances, Spatial sex ratio, and growth) and abundance are still scarce, particularly in the Mediterranean Sea. -
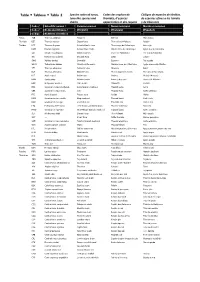
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught.