Iowa State College Journal of Science 16.1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1958: Photopic Field Theory for Natural Hydroso1s
UC San Diego SIO Reference Title Photopic field theory for natural hydroso1s Permalink https://escholarship.org/uc/item/0dk1r645 Author Preisendorfer, Rudolph W Publication Date 1958-09-10 eScholarship.org Powered by the California Digital Library University of California Visibility Laboratory- University of California Scripps Institution of Oceanography San Diego 52, California PHOTIC FIELD THEORY FOR NATURAL HYDROSOLS R. W. Preisendorfer 10 September 1958 Bureau of Ships Index Number NS 714-100 Contract NObs-72092 SIO REFERENCE 58-66 Approved: Approved for Distribution: /£-*.*-'A Seibert Q. Duntley, Director ^—> Roger Revelle, Director Visibility Labora/tory Scripps Institution of Oceanography 1 Photic Field Theory for Natural Hydrosols 2 Rudolph W. Preisendorfer INTRODUCTION The purpose of this note is to present an example of the application of the vector theory of the photic field ("light field") to an important class of scattering-absorbing optical media, namely the class of natural hydrosols consisting, e.g., of oceans, harbors, and lakes. The application is at the same time of practical value in that it yields explicit expressions for the depth-dependence of the light vector in terms of its components at the surface and certain of the optical properties of these media. Furthermore, the discussion presents particularly simple interpretations of the quasipotential and related functions. These interpretations emerge naturally from the geometry and physics of the present application. In this way we add to the evidence that the formalism of the photic field as developed by Moon, Spencer, and others (1), (2), * is of more than academic This paper represents results of research which has been supported by the Bureau of Ships, U. -

A Geometry Oã-Nutrition1
A Geometry oÃNutrition1 PARRY MOON AND DOMINA EBERLE SPENCER Electrical Engineering Department, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, and Mathematics Department, University of Connecticut, Starrs, Connecticut 06268 ABSTRACT The nutritional value of a given sample of food may be specified by a nutrition holor in a fictitious 3-space. This holor possesses magnitude and character. In most cases, we are interested primarily in character, and this can be represented by a point in a 2-space (the nutrition triangle). The triangle allows visualization of re lations among foods and also the addition of nutritional values for a combination of foods. J. Nutr. 104: 1535-1542, 1974. INDEXING KEY WORDS geometry •mathematical model •nutrient patterns Downloaded from The worldwide importance of modern acter of a food may be specified by giving nutritional theory can hardly be overesti any two of the above coordinates. These mated. Nutrition calculations, however, coordinates can be plotted in a nutrition have remained in the arithmetic stage and triangle (fig. 1). have not utilized the possibilities of alge The diagram is advantageous because it braic and geometric formulation. In par shows at a glance the relations among jn.nutrition.org ticular, a geometrization of nutrition al foods: which food has the higher lipid lows one to visualize the relations among content, which has the more protein, etc. nutrients and thus to bypass much of the The triangle can be divided into regions. present routine computation, in accordance Sugars, syrups, and honey are almost pure with the principle that a picture is worth carbohydrate, so they are represented by by on November 25, 2009 a thousand words. -

Views of Farms, Residences, Mills &C., Portraits of Well-Known Citizens, and the Official County Map
Donald Heald Rare Books A Selection of Rare Books Donald Heald Rare Books A Selection of Rare Books Donald Heald Rare Books 124 East 74 Street New York, New York 10021 T: 212 · 744 · 3505 F: 212 · 628 · 7847 [email protected] www.donaldheald.com California 2017 Americana: Items 1 - 34 Travel and Voyages: Items 35 - 58 Natural History: Items 59 - 80 Miscellany: Items 81 - 100 All purchases are subject to availability. All items are guaranteed as described. Any purchase may be returned for a full refund within ten working days as long as it is returned in the same condition and is packed and shipped correctly. The appropriate sales tax will be added for New York State residents. Payment via U.S. check drawn on a U.S. bank made payable to Donald A. Heald, wire transfer, bank draft, Paypal or by Visa, Mastercard, American Express or Discover cards. AMERICANA 1 ADAMS, Ansel Easton (1902-1984) and Mary Hunter AUSTIN (1868-1934). Taos Pueblo. San Francisco: Grabhorn Press, 1930. Folio (17 x 12 1/2 inches). [6] preliminary pages followed by [14]pp. of text. 12 original mounted photographs, printed on Dessonville paper by Ansel Adams, various sizes to 9 x 6 1/2 inches, each with a corresponding caption leaf. Publisher’s tan morocco backed orange cloth, spine with raised bands in six compartments, marbled endpapers (minor fading to the leather). From an edition of 108 numbered copies signed by the author and the photographer, containing magnificent photographs by Ansel Adams. Possibly the most famous of modern photographic works on the West, Taos Pueblo was a collaboration between the young photographer, Ansel Adams, and one of the most evocative writers on the Southwest, Mary Austin. -

Notices of the American Mathematical Society
OF THE AMERICAN MATHEMATICAL SOCIETY VOLUME 16, NUMBER 3 ISSUE NO. 113 APRIL, 1969 OF THE AMERICAN MATHEMATICAL SOCIETY Edited by Everett Pitcher and Gordon L. Walker CONTENTS MEETINGS Calendar of Meetings ..................................... 454 Program for the April Meeting in New York ..................... 455 Abstracts for the Meeting - Pages 500-531 Program for the April Meeting in Cincinnati, Ohio ................. 466 Abstracts for the Meeting -Pages 532-550 Program for the April Meeting in Santa Cruz . ......... 4 73 Abstracts for the Meeting- Pages 551-559 PRELIMINARY ANNOUNCEMENT OF MEETING ....................•.. 477 NATIONAL REGISTER REPORT .............. .. 478 SPECIAL REPORT ON THE BUSINESS MEETING AT THE ANNUAL MEETING IN NEW ORLEANS ............•................... 480 INTERNATIONAL CONGRESS OF MATHEMATICIANS ................... 482 LETTERS TO THE EDITOR ..................................... 483 APRIL MEETING IN THE WEST: Some Reactions of the Membership to the Change in Location ....................................... 485 PERSONAL ITEMS ........................................... 488 MEMORANDA TO MEMBERS Memoirs ............................................. 489 Seminar of Mathematical Problems in the Geographical Sciences ....... 489 ACTIVITIES OF OTHER ASSOCIATIONS . 490 SUMMER INSTITUTES AND GRADUATE COURSES ..................... 491 NEWS ITEMS AND ANNOUNCEMENTS ..... 496 ABSTRACTS OF CONTRIBUTED PAPERS .................•... 472, 481, 500 ERRATA . • . 589 INDEX TO ADVERTISERS . 608 MEETINGS Calendar of Meetings NOn:: This Calendar lists all of the meetings which have been approved by the Council up to the date at which this issue of the c;Noticei) was sent to press. The summer and annual meetings are joint meetings of the Mathematical Association of America and the American Mathematical Society. The meeting dates which fall rather far in the future are subject to change. This is particularly true of the meetings to which no numbers have yet been assigned. -

Hypernumbers and Other Exotic Stuff
HYPERNUMBERS AND OTHER EXOTIC STUFF Photo by Mateusz Dach (1) MORE ON THE "ARITHMETICAL" SIDE Tropical Arithmetics Introduction to Tropical Geometry - Diane Maclagan and Bernd Sturmfels http://www.cs.technion.ac.il/~janos/COURSES/238900-13/Tropical/MaclaganSturmfels.pdf https://en.wikipedia.org/wiki/Min-plus_matrix_multiplication https://en.m.wikipedia.org/wiki/Tropical_geometry#Algebra_background https://en.wikipedia.org/wiki/Amoeba_%28mathematics%29 https://www.youtube.com/watch?v=1_ZfvQ3o1Ac (friendly introduction) https://en.wikipedia.org/wiki/Log_semiring https://en.wikipedia.org/wiki/LogSumExp Tight spans, Isbell completions and semi-tropical modules - Simon Willerton https://arxiv.org/pdf/1302.4370.pdf (one half of the tropical semiring) Hyperfields for Tropical Geometry I. Hyperfields and dequantization - Oleg Viro https://arxiv.org/pdf/1006.3034.pdf (see section "6. Tropical addition of complex numbers") Supertropical quadratic forms II: Tropical trigonometry and applications - Zur Izhakian, Manfred Knebusch and Louis Rowen - https://www.researchgate.net/publication/ 326630264_Supertropical_Quadratic_forms_II_Tropical_Trigonometry_and_Applications Tropical geometry to analyse demand - Elizabeth Baldwin and Paul Klemperer http://elizabeth-baldwin.me.uk/papers/baldwin_klemperer_2014_tropical.pdf International Trade Theory and Exotic Algebras - Yoshinori Shiozawa https://link.springer.com/article/10.1007/s40844-015-0012-3 Arborescent numbers: higher arithmetic operations and division trees - Henryk Trappmann http://eretrandre.org/rb/files/Trappmann2007_81.pdf -

(More) Use Copy Created from Institute Archives Record Copy
MIT Institute Archives & Special Collections. Massachusetts Institute of Technology. News Office (AC0069) From the Office of Public Relations Massachusetts Institute of Technology FOR IMMEDIATE RELEASE Cambridge 39, Massachusetts Telephone: UN 4-6900, ext. 2701-8 The retirement at the end of June of five faculty members at the Massachusetts Institute of Technology was announced by Dr. Julius A. Stratton, President. Marcy Eager, associate professor of electrical engineering, was both teacher and administrator. A graduate from Harvard Engineering School magna cum laude in 1921, he spent the next two decades in industrial posts, concentrating ultimately on various engineering and economic aspects of radio broadcasts. He joined the staff of the M.I.T. Radar School in 1942 and the Electrical Engineering Department in 1946. In addition to teaching the fundamentals of electronic circuits, for many years he has been involved in the administration of the cooperative course in electrical engineering (a 5-year undergraduate course in which students spend part of their time working in industry). He will remain at the Institute on a part-time basis. Professor Eager lives at 75 Abbott Road, Wellesley Hills. Ernest N. Gelotte, associate professor of architecture, has spent his professional career concentrating on the structural aspects of buildings. A graduate of M.I.T. in 1923, he joined the Civil Engineering Department in 1926 and the Architecture Department in 1930. He has always maintained an effective liaison between these Departments. Through his imaginative appreciation of the relation of structures to architecture he has made an outstanding contribution to the teaching program. He will remain at M.I.T. -

THE LIGHT FIELD by A
THE LIGHT FIELD By A. GERSHUN Translated by PARRY MOON and GREGORY TIMOSHENKO Theoretical photometry constitutes a case of "arrested develop ment", and has remained basically unchanged since 1760 while the rest of physics has swept triumphantly ahead. In recent years, how ever, the increasing needs of modern lighting technique have made the absurdly antiquated concepts of traditional photometric theory more and more untenable. A vigorous attempt to bring the theory of light calculation into conformity with the spirit of physics is now in progress. Professor Gershun of the State Optical Institute, Leningrad, is one of the pioneers in this reform, some of his work being contained in the book, "The Light Field"*, a translation of which follows. The purpose of our translation is two-fold: first, to bring to the attention of engineers the new methods of radiometric and photometric calculation; and secondly, to interest mathematicians and physicists in the further development of this branch of field theory. In its present form, the concept of the light vector is of distinct practical value and its general use by illuminating engineers would tend to simplify calcu lations and clarify thinking. Valuable as the methods are, however, they probably do not consti tute the ultimatp solution of the problem. The light field considered in this hook iR a clasRical three-dimensional ypctor field. But the physically important quantity is actually the 1'llumination, which is a function of five independpnt variables, not three. Is it not possible that a more satisfactory theory of the light field could be evolved by use of modern tensor methods in a five-dimensional manifold? We must look to the mathematician for any such development. -

Quarterly of Applied Mathematics
QUARTERLY OF APPLIED MATHEMATICS Vol. XIV APRIL, 1956 No. 1 ON THE CLASSIFICATION OF THE ORDINARY DIFFERENTIAL EQUATIONS OF FIELD THEORY* BT PARRY MOON AND DOMINA EBERLE SPENCER Massachusetts Institute of Technology and University of Connecticut Introduction. Despite the great amount of work that has been done on the solution of the partial differential equations of mathematical physics by separation of variables, the subject remains in an unfinished state. In a comprehensive treatment of the subject, the first problem is to determine separability conditions for the Laplace and Helmholtz equations and to find the coordinate systems that satisfy these conditions. The second problem is to tabulate all the separation equations. The first of these questions has been studied in recent papers [1], while the second is considered in the present contribution. Attempts have been made in the past to subsume all the separation equations under a single general equation; but these formulations have not been satisfactory, since they include equations that are never encountered in physics and they exclude equations that are definitely needed. For instance, the hypergeometric equation includes both Bessel and Legendre equations but excludes most of the separation equations that occur in field theory. There seems to be a widespread idea that B6cher's "generalized Lam 6 equation" with five singularities includes all the needed equations as confluent cases. According to Ince [2], "This systematization was suggested by the discovery of Klein and B6cher that the chief linear differential equations which arise out of the problems of mathematical physics can be derived from a single equation with five distinct regular singular points in which the difference between the two exponents relative to each singular point is §". -

HYPERNUMBERS and OTHER EXOTIC STUFF ( Mathematical Adventure )
HYPERNUMBERS AND OTHER EXOTIC STUFF ( Mathematical Adventure ) Anakin: "I sense Count Dooku." Obi-Wan: "I sense a trap." Anakin: "Next move?" Obi-Wan: "Spring the trap." (1) MORE ON THE "ARITHMETICAL" SIDE Tropical Arithmetics Introduction to Tropical Geometry - Diane Maclagan and Bernd Sturmfels http://www.cs.technion.ac.il/~janos/COURSES/238900-13/Tropical/MaclaganSturmfels.pdf https://en.wikipedia.org/wiki/Min-plus_matrix_multiplication https://en.m.wikipedia.org/wiki/Tropical_geometry#Algebra_background https://en.wikipedia.org/wiki/Amoeba_%28mathematics%29 https://www.youtube.com/watch?v=1_ZfvQ3o1Ac (friendly introduction) https://en.wikipedia.org/wiki/Log_semiring https://en.wikipedia.org/wiki/LogSumExp Tight spans, Isbell completions and semi-tropical modules - Simon Willerton https://arxiv.org/pdf/1302.4370.pdf (one half of the tropical semiring) Hyperfields for Tropical Geometry I. Hyperfields and dequantization - Oleg Viro https://arxiv.org/pdf/1006.3034.pdf (see section "6. Tropical addition of complex numbers") Supertropical quadratic forms II: Tropical trigonometry and applications - Zur Izhakian, Manfred Knebusch and Louis Rowen - https://www.researchgate.net/publication/ 326630264_Supertropical_Quadratic_forms_II_Tropical_Trigonometry_and_Applications Tropical geometry to analyse demand - Elizabeth Baldwin and Paul Klemperer http://elizabeth-baldwin.me.uk/papers/baldwin_klemperer_2014_tropical.pdf International Trade Theory and Exotic Algebras - Yoshinori Shiozawa https://link.springer.com/article/10.1007/s40844-015-0012-3 -

President's Report Issue
MASSACHUSETTS INSTITUTE OF TECHNOLOGY BULLETIN PRESIDENT'S REPORT ISSUE VOLUME 84 NUMBER 1 OCTOBER, 1948 Published by Massachusetts Institute of Technology Cambridge, Massachusetts ---- -- Entered July 3, 1933, at the Post Office, Boston, Massachusetts, as second- class matter under Act of Congress of August 24, 1912. Published by the Massachusetts Institute of Technology, Cambridge Station, Boston, Massachusetts in March, June, October, and November. Issues of the BULLETIN include the reports of the President and of the Treasurer, the General Catalogue, the Summer Session, and the Directory. ---. _Z MASSACHUSETTS INSTITUTE OF TECHNOLOGY BULLETIN President's Report Issue 1947-1948 VOLUME 84 NUMBER I. OCTOBER, 1948 PUBLISHED BY THE INSTITUTE, CAMBRIDGE _ _ _ ______ _·· THE CORPORATION, 1948-1949 President KARL T. COMPTON Secretary Vice President Treasurer WALTER HUMPHREYS JAMES R. KILLIAN, JR. HORACE S. FORD LIFE MEMBERS W. CAMERON FORBES ALFRED L. LooMIS VANNEVAR BUSH EDWIN S. WEBSTER HARLOW SHAPLEY WILLIAM EMERSON PIERRE S. DU PONT ALFRED P. SLOAN, JR. RALPH E. FLANDERS HARRY J. CARLSON LAMMOT DU PONT JAMES M. BARKER GERARD SWOPE FRANK B. JEWETT THOMAS C. DESMOND FRANKLIN W. HOBBS REDFIELD PROCTOR J. WILLARD HAYDEN JOSEPH W. POWELL GODFREY L. CABOT Louis S. CATES WALTER HUMPHREYS WILLIAM C. POTTER MARSHALL B. DALTON VICTOR M. CUTTER BRADLEY DEWEY WILLIAM S. NEWELL ALBERT H. WIGGIN HENRY E. WORCESTER CHARLES E. SPENCER, JR. JOHN R. MACOMBER FRANCIS J. CHESTERMAN ROBERT E. WILSON WILLIS R. WHITNEY SPECIAL TERM MEMBERS Term expires June, 1949 Term expires June, 1951 GEORGE A. SLOAN THOMAs D. CABOT Term expires June, r952 Term expires June 5953 BEAUCHAMP E. -

SURVEY of the LITERATURE on the SOLAR CONSTANT and the SPECTRAL DISTRIBUTION of SOLAR RADIANT FLUX
c/ ? (PAGE& 5 h< (NASA CR OR TMX OR AD NUMBER) SURVEY of the LITERATURE on the SOLAR CONSTANT and the SPECTRAL DISTRIBUTION of SOLAR RADIANT FLUX Thekaekara GPO PRICE S c NASA SP-74 SURVEY of the LITERATURE on the SOLAR CONSTANT and the SPECTRAL DISTRIBUTION of SOLAR RADIANT FLUX M. P. Thekaekara Goddard Space Flight Center Scientific and Technical Information Division 1965 NATIONAL AERONAUTICS AND SPACE ADMINISTRATION Wadhgton, DC. ABSTRACT Data currently available on the solar constant and thed."- spectral dis- tribution of the solar radiant flux are surveyed. The relevant theoretical considerations concerning radiation, solar physics, scales of radiometry and the thermal balance of spacecraft have been discussed briefly. A detailed review has been attempted of the data taken by the Smithsonian Institution, the National Bureau of Standards, and the Naval Research Laboratory, of the methods of data analysis, and the many revisions of the results. The survey shows that the results from different sources have wide discrepancies, that no new experimental data have been taken in recent years, and that the conventional technique of extrapolation to zero air mass leaves large uncertainties. The feasibility of further measurements and of a new method of approach has been discussed in the light of the results of this survey. For sale by the Clearinghouse for Federal Scientific and Technical Information Springfield, Virginia 22151 - Price $2.00 ii CONTENTS INTRODUCTION ..................................... 1 THEORETICAL CONSIDERATIONS ........................ 2 Terminology .................................... 2 Laws of Radiation ................................. 3 Solar Radiant Flux ................................ 5 Solar Simulation and Thermal Balance of Spacecraft .......... 6 Standard Scales of Radiation Measurement ................ 10 ABSORPTION OF SOLAR RADIATION BY THE EARTH'S ATMOSPHERE ............................ -
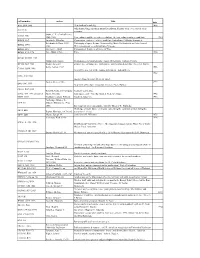
Call Number Author Title Date AG5 .B64 2006 New Book of Knowledge
call number author Title date AG5 .B64 2006 New book of knowledge. 2006 John Simon Guggenheim memorial foundation. Reports of the secretary & of the AS911.J6 treasurer. Snow, C. P. (Charles Percy), AZ361 .S56 1905-1980 Two cultures and the scientific revolution, the two cultures and a second look 1963 BD632 .F67 Formánek, Miloslav. Časoprostor v politice : některé problémy fyzikalismu / Miloslav Formánek. Reichenbach, Hans, 1891- Philosophy of space & time. Translated by Maria Reichenbach and John Freund. BD632 .R413 1953. With introductory remarks by Rudolf Carnap. BD632.G92 Gurnbaum, Adolf Philosophical Problems of Space of Time BD638 .P73 1996 Price, Huw, 1953- Price. 1996 BH301.N3 H55 1985 Hildebrandt, Stefan. Mathematics and optimal form / Stefan Hildebrandt, Anthony Tromba. BT130 .B69 1983 Brams, Steven J. omniscience, omnipotence, immortality, and incomprehensibility / Steven J. Brams. Berry, Adrian, 1937- 1996 CB161 .B459 1996 Next 500 years : life in the coming millennium / Adrian Berry. 1982 E158 .A48 1982 America from the road / Reader's digest. Forbes, Steve, 1947- 1999 E885 .F67 1999 New birth of freedom : vision for America / Steve Forbes. G1019 .R47 1955 Rand McNally and Company. Standard world atlas. G70.4 .S77 1992 oversized Strain, Priscilla. Looking at earth / Priscilla Strain & Frederick Engle. 1992 GB55 .G313 Gaddum, Leonard William, Harold L. Knowles. 1953 Fairbridge, Rhodes W. GC9 .F3 (Rhodes Whitmore), 1914- 2006. Encyclopedia of oceanography, edited by Rhodes W. Fairbridge. Challenge of man's future; an inquiry concerning the condition of man during the GF31 .B68 Brown, Harrison, 1917-1986. years that lie ahead. GF47 .M63 Moran, Joseph M. [and] James H. Wiersma. 1973 Anderson, Walt, 1933- 1996 GN281.4 .A53 1996 Evolution isn't what it used to be : the augmented animal and the whole wired world / Walter Truett Anderson.