Deciphering Groundwater Quality for Drinking and Irrigation in Rural Areas of Paramakudi Block, Tamil Nadu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
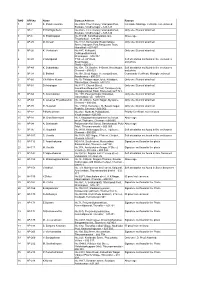
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -
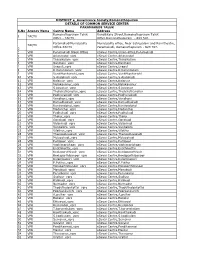
RAMNAD CSC DETAILS (2).Xlsx
DISTRICT e_Governance Society,Ramanathapuram DETAILS OF COMMON SERVICE CENTER PARAMAKUDI TALUK S.No Agency Name Centre Name Address Ramanathapuram Taluk Vandikkara Street,Ramanathapuram Taluk 1 TACTV Office - TACTV Office,Ramanathapuram - 623 501. ParamakudiMunicipality Municipality office, Near aatrupalam and Ravi theatre, TACTV Office-TACTV Paramakudi, Ramanathapuram - 623 707. 2 3 VPR Paramakudi Block Office eSevai Centre,Union office,Paramakudi 4 VPR Arianendal_vprc eSevai Centre,Arianendal 5 VPR Thinaikulam_vprc eSevai Centre,Thinaikulam 6 VPR Nenmeni_vprc eSevai Centre,Nenmeni 7 VPR Urapuli_vprc eSevai Centre,Urapuli 8 VPR K.Karungulam_vprc eSevai Centre,K.Karungulam 9 VPR Venkittankurichi_vprc eSevai Centre,Venkittankurichi 10 VPR S.Andakudi_vprc eSevai Centre,S.Andakudi 11 VPR Kalaiyur_vprc eSevai Centre,Kalaiyur 12 VPR Melakavanur_vprc eSevai Centre,Melakavanur 13 VPR S.kavanur_vprc eSevai Centre,S.kavanur 14 VPR Thelichathanallur_vprc eSevai Centre,Thelichathanallur 15 VPR Podhuvakudi_vprc eSevai Centre,Podhuvakudi 16 VPR Vendhoni_vprc eSevai Centre,Vendhoni 17 VPR Kamudhakudi_vprc eSevai Centre,Kamudhakudi 18 VPR Kanjiendahal_vprc eSevai Centre,Kanjiendahal 19 VPR Madanthai_vprc eSevai Centre,Madanthai 20 VPR Pudhukudi_vprc eSevai Centre,Pudhukudi 21 VPR Tholur_vprc eSevai Centre,Tholur 22 VPR Oorakudi_vprc eSevai Centre,Oorakudi 23 VPR Valankudi_vprc eSevai Centre,Valankudi 24 VPR Vengalore_vprc eSevai Centre,Vengalore 25 VPR Vilathur_vprc eSevai Centre,Vilathur 26 VPR Thenpoduvakudi_vprc eSevai Centre,Thenpoduvakudi 27 VPR -

DEVELOPMENT of SCHEDULE CASTE WOMEN THROUGH SELF HELP GROUPS in PARAMAKUDI TALUK Dr
International Journal of Research in Management, Economics & Commerce (Impact Factor: 4.604, ISSN 2250-057X, Volume 5 Issue 1, January 2015) Website: www.indusedu.org DEVELOPMENT OF SCHEDULE CASTE WOMEN THROUGH SELF HELP GROUPS IN PARAMAKUDI TALUK Dr. M. Meganathan Assistant Professor, Department of Commerce, Alagappa University of Arts and Science College, Paramakudi R. Saravanan Assistant Professor, Department of Commerce, Alagappa University of Arts and Science College, Paramakudi R. Sudha Ganthi Research Scholar, Department of Management Studies, Government Arts College, Paramakudi ABSTRACT Empowerment is a multidimensional process which should enable individual or a group of individuals to realize their full identity and powers in all spheres of life. As per the survey conducted in 2011-2012, the percentage of persons below the Poverty Line in India for the year 2011-12 has been estimated as 25.7% in rural areas, 13.7% in urban areas and 21.9% for the country as a whole. In India Schedule Caste women are not empowered much than compare with any other community people because of that the government was introduced many central and states schemes and given more important for Schedule Caste Women for the purpose of improving the socio-economic status, creation of employment opportunities and development of various skills. This paper focuses to study the socio-economic status of Schedule Caste Women SHGs in Paramakudi Taluk of Ramanathapuram District. Out of 966 SHGs in Paramakudi block 20 SHGs have been randomly selected and then out of 20 SHGs, 120 members have been randomly selected for the field survey, by using multi-stage random sampling method. -

District Census Handbook, Ramanathapuram, Part XII-A & B
CENSUS OF INDIA 1991 SERIES-23 TAMIL NADU DISTRICT CENSUS HAND BOOK RAMANATHAPURAM PART XII A & B VILLAGE AND TOWN DIRECTORY VILLAGE AND TOWNWISE PRiMARY CENSUS ABSTRACT K. SAMPATH KUMAR OF THE INDIAN ADMINISTRATIVE SERVICE DIRECTOR OF CENSUS OPERATIONS TAMILNADU CONTENTS Page No 1. Foreword v-vi 2. Preface vii-ix 3. Distnct Map Facing Page 1 4. Important Statistics 1-2 5. Analytical Note (i) Census concepts: Rural and Urban areas, Census House/Household, SCiST, Literate, 3-5 Main ·Workers, Marginal Workers, Non-workers etc., (Ii) History of ttle District Census Handbook including scope of Village and Town Directory 6-11 and Primary Census Abstract (iii) History of the District and Its Formation, Jurisdictional changes, Location and PhYSiogra phy, Forestry, SOil, Mmerals and mining, Rivers, ElectriCity and power, Land and land use pattern, Agriculture, Irngatlon, Animal husbandry, Fisheries, Industnes, Transport and communication, Climate and rainfall, Education, Health, Places of Tourist and religious im portance. 12-21 6. Brief analysis of the Village and Town Directory and Primary Census Abstract data. 22-37 PART-A Village And Town Directory Section-I Village Directory 39 Note explaining the codes used in the VIII~ge Directory 40-41 1. Tiruvadanai C.D. Block 43 (i) Alphabetical list of villages 44-45 (ii) Village Directory Statement 46-53 2. RaJaslngamangalam C D. Block 55 (i) Alphabetical list of Villages 56-57 (ii) Village Directory Statement 58-67 3. Paramakkudl C D. Block 69 (I) Alphabetical list of Villages 71 (ii) Village Directory Statement 72-77 4. Bogalur C D. Block 79 (i) Alphabetical list of Villages 81 (ii) Village Directory Statement 82-85 5. -

Officials from the State of Tamil Nadu Trained by NIDM During the Year 209-10 to 2014-15
Officials from the state of Tamil Nadu trained by NIDM during the year 209-10 to 2014-15 S.No. Name Designation & Address City & State Department 1 Shri G. Sivakumar Superintending National Highways 260 / N Jawaharlal Nehru Salai, Chennai, Tamil Engineer, Roads & Jaynagar - Arumbakkam, Chennai - 600166, Tamil Nadu Bridges Nadu, Ph. : 044-24751123 (O), 044-26154947 (R), 9443345414 (M) 2 Dr. N. Cithirai Regional Joint Director Animal Husbandry Department, Government of Tamil Chennai, Tamil (AH), Animal husbandry Nadu, Chennai, Tamil Nadu, Ph. : 044-27665287 (O), Nadu 044-23612710 (R), 9445001133 (M) 3 Shri Maheswar Dayal SSP, Police Superintending of Police, Nagapattinam District, Tamil Nagapattinam, Nadu, Ph. : 04365-242888 (O), 04365-248777 (R), Tamil Nadu 9868959868 (M), 04365-242999 (Fax), Email : [email protected] 4 Dr. P. Gunasekaran Joint Director, Animal Animal Husbandary, Thiruvaruru, Tamil Nadu, Ph. : Thiruvarur, Tamil husbandary 04366-205946 (O), 9445001125 (M), 04366-205946 Nadu (Fax) 5 Shri S. Rajendran Dy. Director of Department of Agriculture, O/o Joint Director of Ramanakapuram, Agriculture, Agriculture Agriculture, Ramanakapuram (Disa), Tamil Nadu, Ph. : Tamil Nadu 04567-230387 (O), 04566-225389 (R), 9894387255 (M) 6 Shri R. Nanda Kumar Dy. Director of Statistics, Department of Economics & Statistics, DMS Chennai, Tamil Economics & Statistics Compound, Thenampet, Chennai - 600006, Tamil Nadu Nadu, Ph. : 044-24327001 (O), 044-22230032 (R), 9865548578 (M), 044-24341929 (Fax), Email : [email protected] 7 Shri U. Perumal Executive Engineer, Corporation of Chennai, Rippon Building, Chennai - Chennai, Tamil Municipal Corporation 600003, Ph. : 044-25361225 (O), 044-65687366 (R), Nadu 9444009009 (M), Email : [email protected] National Institute of Disaster Management (NIDM) Trainee Database is available at http://nidm.gov.in/trainee2.asp 58 Officials from the state of Tamil Nadu trained by NIDM during the year 209-10 to 2014-15 8 Shri M. -

List of Food Safety Officers
LIST OF FOOD SAFETY OFFICER State S.No Name of Food Safety Area of Operation Address Contact No. Email address Officer /District ANDAMAN & 1. Smti. Sangeeta Naseem South Andaman District Food Safety Office, 09434274484 [email protected] NICOBAR District Directorate of Health Service, G. m ISLANDS B. Pant Road, Port Blair-744101 2. Smti. K. Sahaya Baby South Andaman -do- 09474213356 [email protected] District 3. Shri. A. Khalid South Andaman -do- 09474238383 [email protected] District 4. Shri. R. V. Murugaraj South Andaman -do- 09434266560 [email protected] District m 5. Shri. Tahseen Ali South Andaman -do- 09474288888 [email protected] District 6. Shri. Abdul Shahid South Andaman -do- 09434288608 [email protected] District 7. Smti. Kusum Rai South Andaman -do- 09434271940 [email protected] District 8. Smti. S. Nisha South Andaman -do- 09434269494 [email protected] District 9. Shri. S. S. Santhosh South Andaman -do- 09474272373 [email protected] District 10. Smti. N. Rekha South Andaman -do- 09434267055 [email protected] District 11. Shri. NagoorMeeran North & Middle District Food Safety Unit, 09434260017 [email protected] Andaman District Lucknow, Mayabunder-744204 12. Shri. Abdul Aziz North & Middle -do- 09434299786 [email protected] Andaman District 13. Shri. K. Kumar North & Middle -do- 09434296087 kkumarbudha68@gmail. Andaman District com 14. Smti. Sareena Nadeem Nicobar District District Food Safety Unit, Office 09434288913 [email protected] of the Deputy Commissioner , m Car Nicobar ANDHRA 1. G.Prabhakara Rao, Division-I, O/o The Gazetted Food 7659045567 [email protected] PRDESH Food Safety Officer Srikakulam District Inspector, Kalinga Road, 2. K.Kurmanayakulu, Division-II, Srikakulam District, 7659045567 [email protected] LIST OF FOOD SAFETY OFFICER State S.No Name of Food Safety Area of Operation Address Contact No. -

Ramnathapuram - District Agricultural Plan
Ramnathapuram - District Agricultural Plan Wrapper Project team Preface Foreword Executive Summary Chapter I Chapter II Chapter III Chapter IV Chapter V Chapter VI Meeting Proceedings Photos Paper cuttings NATIONAL AGRICULTURAL DEVELOPMENT PROGRAMME (NADP) DISTRICT AGRICULTURE PLAN RAMANATHAPURAM DISTRICT Centre for Agricultural and Rural Development Studies (CARDS) Tamil Nadu Agricultural University Coimbatore – 641 003 2008 NATIONAL AGRICULTURE DEVELOPMENT PROJECT – DISTRICT AGRICULTURE PLAN PROJECT TEAM Overall Coordination : Dr. K. Palanisami, Director, CARDS and Nodal Officer (NADP) Dr. R. Venkatram, Professor and Principal Coordinator (NADP) District Level : Dr.S.Senthilnathan Coordination Associate Professor Dept.of Agricultural Economics TNAU, Coimbatore-3 Thiru.G.Mahalingam Joint Director of Agriculture Ramanathapuram Dr.G.Srinivasan Associate Professor and Head Coastal Salinity Research Centre Ramanathapuram Dr.A.Sakunthalai Associate Professor (Agrl. Extension) Krishi Vigyan Kendra Coastal Salinity Research Centre Ramanathapuram Tamil Nadu Agricultural University Prof. C.RAMASAMY COIMBATORE-641 003 Vice-Chancellor TAMIL NADU INDIA. FOREWORD Date ........................... The National Development Council resolved that Agricultural Development strategies must be reoriented to meet the needs of farmers and called upon the Central and State governments to evolve a strategy to rejuvenate agriculture with a commitment to achieve four per cent annual growth in the agricultural sector during the 11th plan. The council also recommended special Additional Central Assistance Scheme named National Agriculture Development Programme (NADP) be launched. To implement this, formulation of District level action plans is the pre-requisite and thus District Agriculture Plan of various districts in Tamil Nadu has been prepared with the financial assistance of Government of India. The task of preparing the District Agriculture Plan has been given to Tamil Nadu Agricultural University by Government of Tamil Nadu. -

District Elementary Education Plan Perspective Plan Ramanathapuram District Tamil Nadu
SARVA SHIKSHA ABHIYAN DISTRICT ELEMENTARY EDUCATION PLAN DEEP PERSPECTIVE PLAN 2001 - 2010 RAMANATHAPURAM DISTRICT TAMIL NADU ilERARY T' CHZTIL Maticr-' ’cr.ira-.’ ci ii -;'r--:iona| Pbni; :' iu^ . i 17-13.'... ;.c:o Mats. New I 0)6 DOC, N o...........................— .— 3 _______________ —W DfS I'KlCr MAI* - IMMANA'filAPMRAM RAMAHATHAPURAM DISTRICT Name of the Blocks 1. Ramanaihapiirani 6. Paraniakiidi 2. I'liiru ppullani 7. Bogalur 3. Mandapam 8. Nainarkoil Id 4 . R.S.Mangaiani 9. Mudukula(hu.‘ 5. ThiruNadanai 10. KamuthI II. Kadaladi 1.6.1. Present Status of Indicators The present status of the indicators for elementary classes in the year 2001-2002 is noted below. 1. Gross Access Ratio - 97.33% 2. Gross Enrolment Ratio - 95.40% 3. Net Enrolment Ratio - 92.79% 4. Completion Rate - 64.50% 5. Repetition Rate - 19.00% 6. Dropout Rate - 16.50% Appropriate strategies and activities have been planned to reach the objectives on the basis of indicators. 1.6.2. District SSA Target The proposed target to the objectives by indicators of SSA during the plan period is as follows: S. Indicators 2001- 2002- 2003- 2004- 2005- ^ 0 ^ 2007- 2^08- 2009- No »02 ’03 ’04 ’05 ’06 ’07 ’08 ’09 ’10 1 GAR 97.33 100 100 100 100 100 100 100 100 2 GBR 95.40 100 100 100 100 100 100 100 100 3 NER 92.79 100 100 100 100 100 100 100 100 4 CR 64.50 68 75 85 90 100 100 100 100 5 RR 19.00 17 15 10 5 0 0 0 0 6 DR 16.50 15 10 5 5 0 0 0 0 7 Achievement 42 50 60 68 75 80 85 90 95 Level The target of 100% access to elementary will be achieved by 2002-’03. -

2017 DHAN Foundation Water Matters
Water Matters Annual Report 2017 DHAN Foundation Water Matters Annual Report 2017 DHAN Foundation Contents 5 Abbreviations & Acronyms 7 Introduction to DHAN Foundation 11 Water Matters Perspectives, Principles and Practices of DHAN’s Water Initiatives Advanced Themes 53 Kalanjiam Community Banking 64 Vayalagam Tankfed Agriculture Development Themes 68 Coastal Conservation and Livelihoods 72 Rainfed Farming Development 75 Sustainable Healthcare Advancement 79 Social Security for Building Resilience 83 Working with Panchayats 86 Tata-Dhan Academy 89 Information and Communication Technology for Poor 92 DHAN - Karunai Illam 94 New Themes 98 Centres for Integration 101 Impact 123 Financial Statements 136 Acknowledgement 139 Board of Trustees 142 DHAN Foundation - Addresses Abbreviations & Acronyms ACEDRR Advanced Centre for Enabling Disaster Risk Reduction ASKMI Advanced Centre for Skill and Knowledge on Mutual Insurance BCC Behaviour Change Communication CALL Coastal Conservation and Livelihoods CDA Cluster Development Association CIT4D Communication and Information Technology for Development CURE Centre for Urban Water Resources DEWATS Decentralised Wastewater Treatment Systems DHAN Development of Humane Action DPA DHAN People Academy DRC DHAN Resource Centre KDFS Kalanjiam Development Financial Services KRCH Kalanjiam Reproductive and Child Health KTL Kalanjium Thozhilagam Limited LIFE Livelihood Initiative with Functional Education MAVIM Mahila Arthik Vikas Mahamandal MDG Millennium Development Goal MFG Microfinance Group MGNREGS Mahatma -

Ramanathapuram 9843050767 TAFE 9500 4WD, PTO HP 56.40 1
AGRICULTURAL ENGINEERING DEPARTMENT The Beneficiary details of Custom Hiring Centres established so far with subsidy assistance of SMAM and NADP Scheme from the year 2014-15 to 2020-21 Sl. Name of Beneficiary Address of Custom Hiring Centre Name of Block Mobile No/Phone Name of Agrl.Machinery available in the centre Qty No. No. 1 P.Ponnusamy Selvanayaga Muthukulathur 98659 40433 Tractor 55 HP 1 puram, Muthukulathur taluk Tractor 40 HP 1 Rotavator 2 Zero Seed Drill 2 Baler 1 5 Tyne Cultivator 1 9 Tyne Cultivator 1 Multi Crop Thresher 1 Power weeder/ intercultivator 1 2 T.Kamalahasan Kallidiyendal, Nainarkoil 98431 12011 Tractor 41.6 HP 1 Nainarkoil Tractor 43.4 HP 1 MB Plough 2 9 tyne Cultivator 2 5 tyne Cultivator 2 Rotavator 2 Baler 1 2 Row Disc Plough 1 3 Row Disc Plough 1 Seed Drill 2 Power Weeder 1 3 C.Kothanda Mandala Kamuthi 98433 77519 Tractor- 36.8 PTO HP 1 ramalingam manickam, Kamuthi Tractor-41.20 PTO HP 1 Reversible MB Plough 1 3 Furrow Disc Plough 1 Rotavator- 42 Blade 1 Rotavator- 42 Blade 1 Seed Drill 1 Multi Crop Thresher 2 9 Tyne Cultivator 1 AGRICULTURAL ENGINEERING DEPARTMENT The Beneficiary details of Custom Hiring Centres established so far with subsidy assistance of SMAM and NADP Scheme from the year 2014-15 to 2020-21 Sl. Name of Beneficiary Address of Custom Hiring Centre Name of Block Mobile No/Phone Name of Agrl.Machinery available in the centre Qty No. No. 4 K.Kalimuthan Sevvoor, Bogalur 94423 21953 Tractor - PTO HP : 39.20 HP 2 Bogalur Rotary Tiller 2 Two furrow disc plough 1 Three furrow disc plough 1 5 Tyne Cultivator 2 9 Tyne Cultivator 2 Baler 1 Post hole digger 1 RotoSeed drill 2 Power Weeder 1 Inter cultivater 1 Mobile shredder 2 5 K.Thirumaran Thelichatha Paramakudi 99655 25964 Tractor - PTO HP : 52.90 HP 2 nallur, Paramakudi Reversible plough 2 Post hole digger 1 Rotavator - 42 blade 2 Mobile shredder 1 Power tiller 2 6 A.Thirumurugan Alankulam, Nallirukkai Post, Thiruppullani block. -

Annual Work Plan & Budget
SARVA SHIKSHA ABHIYAN DISTRICT ELEMENTARY EDUCATION PLAN ANNUAL WORK PLAN & BUDGET 2003 - 2004 nilNIEPA DC □12172 RAMANATHAPURAM DISTRICT TAMILNADU SARVA SHIKSHA ABHIYAN TAMILNADU RAMANATHAPURAM 1)1 STRICT ANNUAL WORK PLAN AND BUDGET 2002 - 2003 Atiom' H e ai«- ' i= '.1 ' i : a i n i s t r » t i © a . M-iit-is T)-i:?V72 30C , No - .... ...............................- Of' CONTENTS CHAPTER TITLE PAGE No. 1. Plan Over View 07 2. Progress Review 34 3. Activities and Budgets 84 4. Annexures 99 CHAPTER -1 CONTENTS CHAPTER No TITLE PAGE No. 1.1 Introduction 07 1.2 Planning Process 08 1.3 General Profile 10 1.4 Liducational Profile 12 1.5 liai'ly childhood care and Education 24 1.6 Out of School children 25 1.7 Special Focus Group 26 1.8 VECs, CRCs, BRCs 27 1.9 Infrastructure 28 1.10 District Project Office 31 CHAPTER -1 TABLES TABLE No. TITLE PAGE No. L Population 10 ' 2. Literacy Rate 11 J. Number of Schools 12 4. Number of Teachers 13 5. Number of Pupils 14 6. Number of Pupils - Boys & Girls 14 7. GAR - Primary & Upper Primary 15 8. GER&NER 16 9. Enrolment - Primary 17 10. Enrolment - Upper Primary 17 11. Disabled Children 18 12. CR, RR, DR - Primary 19 Tk C'R, KK,I^R-Upper Primary 20 14. C’K, RR, DR-SC7 ST 21 15. Transition Rate 23 16. Teacher Pupil Ratio 24 17. ECCE 25 18. Special Focus Group 27 19. BRC, CRC, & VEC 28 20. Three Class Rooms 30 21. Two Class Rooms 30 INDJA Stales .ind IJriion icrritonc;. -

Assessment and Reassessment of Secondary and Micronutrient Status
International Journal of Chemical Studies 2017; 5(5): 902-904 P-ISSN: 2349–8528 E-ISSN: 2321–4902 IJCS 2017; 5(5): 902-904 Assessment and reassessment of secondary and © 2017 IJCS Received: 08-07-2017 micronutrient status between 1980-2000 and Accepted: 09-08-2017 effect of garden land environment on soil Aswathy S Nair properties and availability of micronutrients in Department of Soil Science, Tamil Nadu Agricultural Ramanathapuram district of Tamil Nadu using University (TNAU), Coimbatore, Tamil Nadu, India GPS and GIS technology S Thyigeshwari Department of Soil Science, Tamil Nadu Agricultural Aswathy S Nair, S Thyigeshwari and D Muthumanickam University (TNAU), Coimbatore, Tamil Nadu, Idina Abstract Totally 1820 geo-referenced surface soil samples covering 455 panchayat villages in 11 blocks of D Muthumanickam Ramanathapuram district were collected to assess the available sulphur and micro nutrient status in soils Department of Soil Science, besides to create a data bank on soil fertility status including multi nutrient status. The study involved Tamil Nadu Agricultural University (TNAU), Coimbatore, assessment and reassessment of multinutrient and single nutrient 1980-2000. From the present Tamil Nadu, India investigation, it is revealed that ZnCuB; FeZnCu and FeZnB was observed as three nutrient deficiencies in Ramanathapuram district with a mean of 17.9, 0.3 and 0.2 per cent respectively. Higher per cent of three nutrient deficiencies viz., ZnCuB was observed in Thiruvadanai block and lower per cent was observed in Nainarkovil. ZnCu registered the highest percentage of two nutrient deficiencies. Maximum individual deficiency of Zn, Cu and B nutrients observed as single nutrient deficiency with a mean of 26.4, 5.7 and 4.6 per cent respectively.