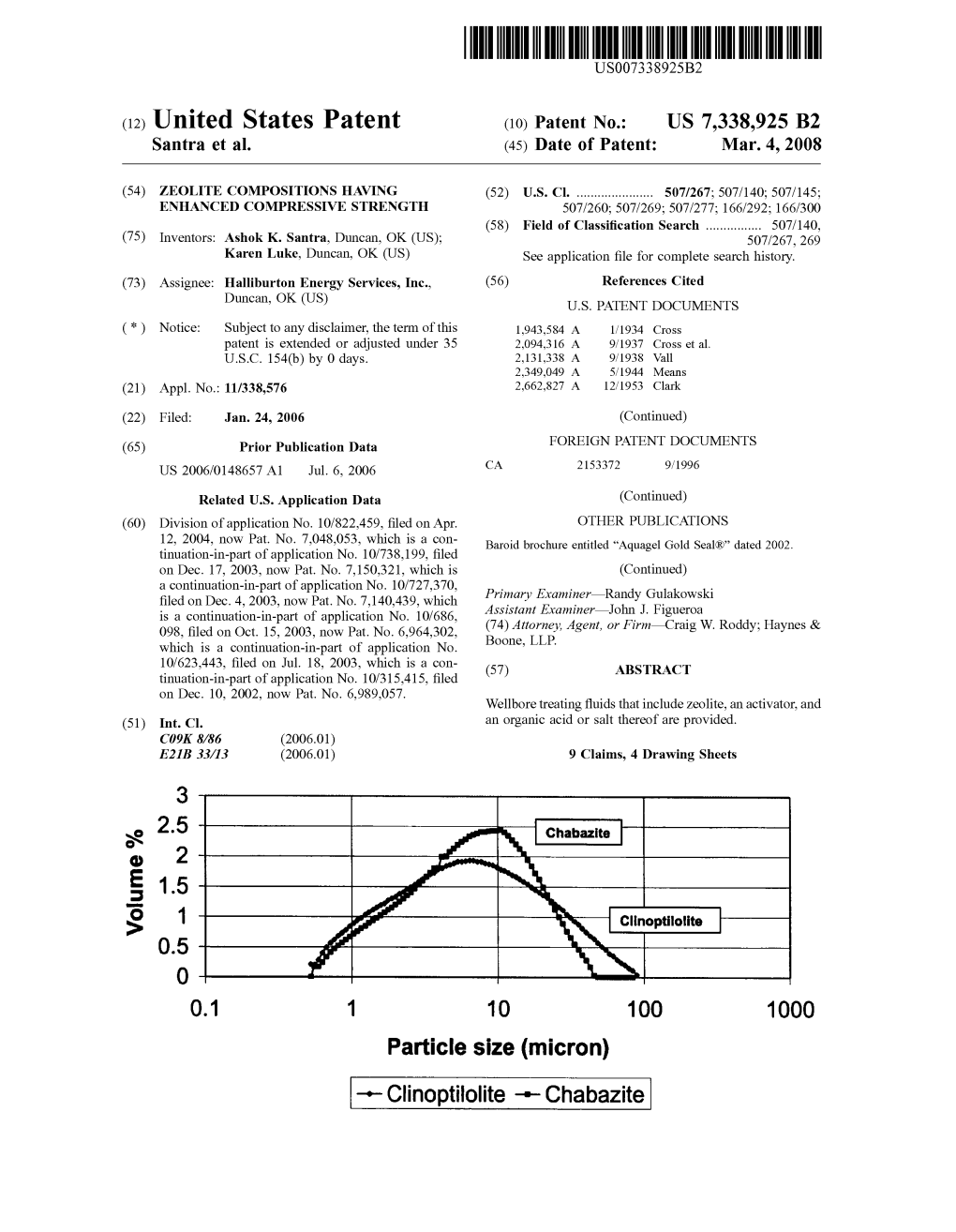
Particle Size (Micron) -- Clinoptilolite -- Chabazite US 7,338,925 B2 Page 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adsorption of Analcime and Zsm-5 on Metals
1 Wang Yuxian ADSORPTION OF ANALCIME AND ZSM-5 ON METALS Thesis CENTRAL OSTROBOTHNIA UNIVERSITY OF APPLIED SCIENCES Degree Programme in Chemistry and Technology December 2011 2 ABSTRACT CENTRAL OSTROBOTHNIA Date Author UNIVERSITY OF APPLIED SCIENCES Technology and Business December 2011 Yuxian Wang Degree programme Chemistry and Technology Name of thesis ADSORPTION OF ANALCIME AND ZSM-5 ON METALS Instructor Pages Laura Rahikka 44 + Appendices (2) Supervisor Laura Rahikka and Maija Rukajärvi-Saarela Natural zeolite with the basic cell structure of AlO4 and SiO4 tetrahedron has the ability of sewage purification. The way to adsorb in sewage is similar to activated carbon, and the zeolite has a good capacity of adsorption on cations. After modification, the activated zeolite acquires a better capacity of adsorption, cation-exchange and ion-exchange. After surfactant modification, zeolite can absorb the anions and organic compounds. In this way, zeolite can adsorb many kinds of ions in sewage. The aims of the thesis were: study the properties of natural zeolites; the adsorption capacity of analcime and ZSM-5 on metals and the theory of modification. In the experiment, the aim was to check out the adsorption capacity of Cu2+ and Ni2+ by using analcime and ZSM-5 and make a compare of the adsorption capacity between the cations in the same zeolite and between the zeolites in the same cations to find out which zeolite is better and in the same zeolite which cation was better adsorbed. The results of the experiment were that the Cu2+ cation was adsorbed better than Ni2+ in both analcime and ZSM-5. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Mineralogical Characterization of Paulingite from Vinarick I Hora, Czech Republic
Mineralogical characterization of paulingite from Vinarick i Hora, Czech Republic C. L. LENGAUER, G. GIESTER AND E. TILLMANNS Institut fiir Mineralogie und Kristallographie, Universit~it Wien - Geozentrum, Althanstrasse 14, A-1090 Wien, Austria Abstract A sample of the zeolite paulingite from the locality Vinarick~i Hora was investigated by means of chemical, thermal, powder and single crystal X-ray methods. The fully transparent, colourless to pale yellow crystals exhibit the form {110} and occur together with phillipsite. The chemical composition is (Cae.57K2.zsBal.39Nao.3s)(Alll.5sSi30.59084)'27HeO, Z = 16 with minor amounts of Mg (<0.05), Sr (<0.13), Mn (<0.01), and Fe (<0.04). The chemical differences from previously described paulingites are a high Ba- content, a lower Si/(AI+Fe) ratio of 2.64, and a reduced water-content. The calculated density is 2.098 g cm -3, and the observed refractive index is 1.482(2). The dehydration behaviour is characterized by a main weight loss from 24-190~ (-11.2 wt.%, ~ 21HeO) and a minor weight loss from 190-390~ (-3.1 wt.%, -~ 6H20). The rehydration capability was proven up to 150~ The dehydration process during the main weight loss is accompanied by a reduction of the cell volume of 11%. The refined lattice parameters of the X-ray powder data are a = 35.1231 (5) A and a = 33.7485(8) ,~ of an untreated and a dehydrated sample, respectively. A breakdown of the paulingite structure can be observed while the remaining water content decomposes. The single crystal X-ray refinement of this chemically different sample material derived three main cation positions, which are inside a so called paulingite or 7t-cage (Ca), between 8-rings of neighbouring n-cages (Ba), and in the centre of the non-planar 8-rings of the 7-cage (K). -

The Characterization of Indonesian's Natural Zeolite for Water Filtration System
Valensi Vol. 3 No. 2, November 2013 (129-137) ISSN : 1978 - 8193 The Characterization of ,ndonesian‘s Natural Zeolite For Water Filtration System Mirzan T. Razzak1,2*, Thamzil Las2, Priyambodo1 1Center for Integrated Laboratory, 2Faculty of Science and Technology State Islamic University of Syarif Hidayatullah Jakarta 15412 *Email: [email protected] Abstract The characterization of ,ndonesian‘s natural zeolite for water purification has been done. The objective is to obtain a general guidance for development of natural zeolite in molecular sieves, ion exchange and catalyst applications. The zeolites originated from Lampung (ZL) was characterized by using XRD. It was found that the zeolites indicate belong to clinoptilatite and mordenite groups, respectively. The crystal system for ZL was monoclinic with end-centered lattice and space group of Cm/2 (12). The crystal system for ZB was orthorhombic with end- centered lattice and space group of CmC21 (36). The character of both zeolites were confirm by ICDD standard library. It was also found that the particle size of zeolites did not affect the XRD spectra where zeolite of 40-50 mesh was similar to that XRD spectra of zeolite of 170-200 mesh. The chemical analyses show that both zeolites contain almost similar chemical elements. The contents of Fe, Ca, and K were found higher in ZL as it is compared to ZB. Reciprocally, Na was found higher in ZB. The water filtration ability shows that ZB was better than ZL for filtering of Pb and Fe elements. These ability were improved by chemical activation of zeolites. These two zeolites, howover, behave similar less ability for the filtering of Ca and Mg elements due to that elements probably were originally exist in the zeolite structure. -

Geology of Natural Zeolites and Zeolitic Rocks
Pure &Appl.Chern., Vol.52, pp.2II5—2l3O. Pergamon Press Ltd. 1980. Printed in Great Britain. PlenaryPaper — Geology and Mineralogy GEOLOGY OF NATURAL ZEOLITES AND ZEOLITIC ROCKS Azumalijima Geological Institute, Faculty of Science, University of Tokyo, 7-3-1 Hongo, Tokyo 113, Japan ABSTRACT Presentstatus, particularly development in the latest decade, of the geology of natural zeolites and zeolitic rocks has been overviewed. Emphases are focused on the classification of genetical occurrences and the synthesis deduced from the nature.. INTRODUCTION Nearly two centuries have passed since zeolite was discovered and named by Cronstedt in 1756. Collection and mineralogical description of pretty, coarse crystals were the primary studies of zeolites for a long time till early this century. It is a great monument that the concept of zeolite facies was established by Eskola in 1936 as the low— est grade of the metamorphic facies. The concept was greatly developed by Coombs in New Zealand, having further been visualized by lijima, Seki and Utada in Japan. Researches on zeolites in alkaline, saline lake deposits by Hay, Gude and Sheppard in USA made our eyes open to the zeolite formation at or near the Earth's surface. From deep—sea sediments zeolites were already reported by Murray and Renard in 1891. The Deep Sea Drilling Project (1968—1975) by the Glomar Challenger cruises shed light on the deep—sea zeolites. Our knowledge on natural zeolites and zeolitic rocks has rapidly expanded during the latest two decades. The growth of study on natural zeolites has been and will be stimulated by increasing needs of them as a potential natural resource, instead of synthetic zeolites, particularly in Africa, East and West Europe, Japan, USA and USSR. -

Distribution and Genesis of Authigenic Silicate Minerals in Tuffs of Pleistocene Lake Tecopa, Inyo County California
Distribution and Genesis of Authigenic Silicate Minerals In Tuffs of Pleistocene Lake Tecopa, Inyo County California GEOLOGICAL SURVEY PROFESSIONAL PAPER 597 Distribution and Genesis of Authigenic Silicate Minerals In Tuffs of Pleistocene Lake Tecopa, Inyo County California By RICHARD A. SHEPPARD and ARTHUR J. GUDE 3d GEOLOGICAL SURVEY PROFESSIONAL PAPER 597 Zeolites, potassium feldspar, searlesite and clay minerals formed during diagenesis of rhyolitic vitric tuffs that were deposited in a saline lake UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1968 UNITED STATES DEPARTl\fENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. GS 68-196 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 -Price 40 cents (paper cover) CONTENTS Page Page Abstract __________________________________________ _ 1 Authigenic minerals-Continued Introduction--------------------------------------- 1 Erionite ___ ------ __ --------- __ - ----- ____ ------- 15 Location ______________________________________ _ 1 Opal---------------------------------------~-- 16 Geography ____________________________________ _ 1 Phillipsite_________________________ ---_____ ·--_- 16 Previous work _________________________________ _ 2 Potassium feldspar _____________________________ _ 18 Name of the lake ______________________________ _ 2 Searlesite ________________ -- _______ ------------- 18 , Scope of investigation __________________________ _ 2 Diagenetic -

1 Critical Review on Clinoptilolite Safety and Medical Applications in Vivo 1
1 Critical review on clinoptilolite safety and medical applications in vivo 2 Sandra Kraljević Pavelić1*, Jasmina Simović Medica2, Darko Gumbarević1, Ana Filošević, 3 Nataša Pržulj3, Kresimir Pavelic1,4, 4 5 1 University of Rijeka, Department of Biotechnology, Centre for High-throughput 6 technologies, Radmile Matejčić 2, 51000 Rijeka, Croatia 7 2 General Hospital Pula, Negrijeva 6/Zagrebačka 30, 5100 Pula, Croatia 8 3 University College London, Computer Science Department, London, WC1E 6BT, UK 9 4 Juraj Dobrila University of Pula, Zagrebačka 30, 52100 Pula, Croatia 10 Correspondence: 11 Professor Sandra Kraljević Pavelić, University of Rijeka, Department of Biotechnology, 12 Centre for High-throughput technologies, Radmile Matejčić 2, 51000 Rijeka, Croatia, 13 [email protected], 14 15 E-mail addresses: 16 Jasmina Simović Medica [email protected] 17 Darko Gumbarević [email protected] 18 Ana Filošević [email protected] 19 Kresimir Pavelic [email protected], [email protected] 20 Nataša Pržulj [email protected] 21 1 22 Abstract: 23 Unique and outstanding physical and chemical properties of zeolite materials have made them 24 extremely useful in a variety of applications including agronomy, ecology, manufacturing and 25 industrial processes. Recently, a more specific application of one naturally occurring zeolite 26 material, clinoptilolite, has widely been studied in veterinary and human medicine. Due to a 27 number of positive effects on health, including detoxification properties, usage of clinoptilolite- 28 based products in vivo increased enormously. However, concerns have been raised in the 29 public of the safety of clinoptilolite materials for in vivo applications. Here, we review the 30 scientific literature on the health effects and safety in medical applications of different 31 clinoptilolite-based materials and propose some comprehensive, scientifically-based 32 hypotheses on possible biological mechanisms underlying observed effects on the health and 33 body homeostasis. -

The Genesis of Zeolites
Eur. J. Mineral. 1989,1,479-487 The genesis of zeolites GLAUCoGOTTARDIt* Istituto di Mineralogia e Petrologia, Università di Modena, via S. Eufemia 19,1-41100 Modena, Italy Abstract: The equilibrium diagrams of zeolites and the different possibilities of synthesizing zeolites starting from chemicals, minerals, and natural glasses are reviewed so to have a general picture of the conditions of crystallization of these minerals. Subsequently, a description and interpretation is given of the geological environments where zeolites crystallize in nature. Key-words: zeolite, diagenesis, very-low-grade metamorphism, hydrothermalism, volcanic glass. 1. Introduction and heulandite generally contain some M+ ca tions, which are almost absent in laumontite, yu This topic has been the subject of so many publi gawaralite and wairakite, so the alkali metal con cations (e.g. Hay, 1978, 1986; Iijima, 1978, 1980; centration in the system may influence the given Kastner & Stonecipher, 1978; Surdam & Shep- boundaries. Additional diagrams on these zeolites pard, 1978) over the last ten years, that one may can be found in the literature, but none is known wonder "Why another one?". As a matter of fact, to the author for zeolites other than those men all these previous studies give detailed informa tioned here. Field and laboratory evidence suggests tion on rock-forming zeolites, generally crystal that some other alkali zeolites may have a stabil lized from natural glasses during diagenesis, but ity field; this is certainly true for clinoptilolite, they omit any consideration of zeolites in veins the siliceous alkali-rich variant of heulandite, and and vugs of massive rocks. The author also aims is also probably true for natrolite and mordenite. -

Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR)
chemengineering Article Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR) Muhammad Yusuf Prajitno 1,* , Mohamad Taufiqurrakhman 2 , David Harbottle 1 and Timothy N. Hunter 1,* 1 School of Chemical and Process Engineering, University of Leeds, Leeds LS2 9JT, UK; [email protected] 2 School of Mechanical Engineering, University of Leeds, Leeds LS2 9JT, UK; [email protected] * Correspondence: [email protected] (M.Y.P.); [email protected] (T.N.H.); Tel.: +44-(0)113-343-2790 (T.N.H.) Abstract: Natural clinoptilolite was studied to assess its performance in removing caesium and stron- tium ions, using both static columns and an agitated tube reactor (ATR) for process intensification. Kinetic breakthrough curves were fitted using the Thomas and Modified Dose Response (MDR) mod- els. In the static columns, the clinoptilolite adsorption capacity (qe) for 200 ppm ion concentrations was found to be ~171 and 16 mg/g for caesium and strontium, respectively, highlighting the poor material ability to exchange strontium. Reducing the concentration of strontium to 100 ppm, however, led to a higher strontium qe of ~48 mg/g (close to the maximum adsorption capacity). Conversely, halving the column residence time to 15 min decreased the q for 100 ppm strontium solutions e to 13–14 mg/g. All the kinetic breakthrough data correlated well with the maximum adsorption Citation: Prajitno, M.Y.; capacities found in previous batch studies, where, in particular, the influence of concentration on the Taufiqurrakhman, M.; Harbottle, D.; slow uptake kinetics of strontium was evidenced. -

Synthesis of a Heulandite-Type Zeolite by Hydrothermal Conversion of Zeolite Pl
Synthesis of a heulandite-type zeolite by hydrothermal conversion of zeolite Pl Shervin Khodabandeh and Mark E. Davis* Department of Chemical Engineering, California Institute of Technology, Pasadena, CA 91 125, USA A synthetic analogue of the zeolite heulandite is elemental analysis). The powder X-ray diffraction pattern of synthesized by hydrothermal treatment of zeolite P1 with this solid is shown in Fig. l(a). solutions containing CaClz and NaOH. Since perlite glass is a natural material, it contains impurities similar to those found in natural heulandite. Therefore, we Heulandite-type zeolites (heulandite and clinoptilolite) are the developed a synthesis route to heulandite that utilized pure most abundant natural zeolites; in spite of this, their synthesis in starting reagents. Zeolite P1 was prepared from an amorphous the laboratory has been extremely difficult. A natural calcium- aluminosilicate gel having the composition A1203: 9.4 dominant zeolite, heulandite possesses a two-dimensional pore Si02:3.7 Na20:x H20 as follows. Two grams of sodium structure consisting of intersecting channels in distinct crys- aluminate (from EM) were dissolved in 60 ml of distilled water tallographic directions bounded by eight and ten tetrahedral and 4 g of a SO% m/m solution of NaOH were added to this atoms.' Heulandite zeolites have Si/Al ratios between 2.8 and 4. solution. The solution was stirred while 5.20 g of Cab-0-Sil Clinoptilolite is a heulandite-type zeolite that has an Si/Al ratio (grade M5) were slowly added. The resulting gel was stirred at > 4, and a sodium and potassium content such that (Na + K) > room temp. -

Gravimetric Adsorption Measurements of Helium on Natural Clinoptilolite and Synthetic Molecular Sieves at Pressures up to 3500 K
Gravimetric adsorption measurements of helium on natural clinoptilolite and synthetic molecular sieves at pressures up to 3500 kPa Arash Arami-Niya, Thomas E. Rufford*, Greg Birkett, Zhonghua Zhu School of Chemical Engineering, The University of Queensland, St Lucia 4072 Australia *Corresponding author: [email protected] Abstract We report helium adsorption capacities and the true specific impenetrable solid volumes of a clinoptilolite-rich Escott zeolite from Werris Creek (Australia), synthetic 3A and 4A zeolites, and carbon molecular sieve 3K-172 measured by a gravimetric method at pressures of (300 - 3500) kPa and temperatures in the range of (303 - 343) K. Our helium adsorption procedure extends the previous works by Gumma and Talu [1] to determine the impenetrable solid volume of the adsorbent, which in standard helium pycnometry is determined under the assumption that helium does not adsorb at room temperature. Our results confirm helium adsorption on these solids is small, but not zero: equilibrium helium adsorption capacities measured at 3500 kPa and 303 K were 0.067 mmol/g on Escott, 0.085 mmol/g on 3A, 0.096 mmol/g on 4A and 0.089 mmol/g on 3K-172. The specific solid volumes determined by the Gumma and Talu method were 10 – 15 % larger than the specific solid volumes measured by standard helium pycnometry, and this error can result in uncertainties of 2.6 – 28 % in the equilibrium adsorption capacities of CO2 and N2 measured at high pressures. The uncertainties were largest for N2 on the Escott zeolite, which had the lowest equilibrium adsorption capacity for N2. -

A Review: Insecticidal Potential of Zeolite (Clinoptilolite), Toxicity Ratings and General Properties of Turkish Zeolites
11th International Working Conference on Stored Product Protection A review: Insecticidal potential of Zeolite (Clinoptilolite), toxicity ratings and general properties of Turkish Zeolites Eroglu, N.*# The Scientific and Technological Research Council of Turkey, Food Institute, Gebze, Kocaeli, Turkey *Corresponding author, Email: [email protected], [email protected] #Presenting author, Email: [email protected], [email protected] DOI: 10.14455/DOA.res.2014.116 Abstract Zeolites are microporous crystalline aluminosilicates derived from the reaction of volcanic rocks, ash layers and an alkaline groundwater. Atlas of Zeolite Framework Types currently recognizes about 218 framework Zeolite structures. Since its first discovery in 1756 by Swedish mineralogist A.F. Cronstedt, over 40 natural zeolites are listed. Non-toxicity (IARC, 1997a,b) and safety for human consumption (FDA GRAS Listings, 2006) of natural zeolites accelerate recognition and expand the applications of agricultural uses. Furthermore, Codex Alimentarius Commission (1999) endorses pest control in food commodities and lists Zeolite as granted substance in organic food production and in plant protection. Public awareness and growing concern to environmental issues encourage new approaches of alternative ecofriendly methods such as inert dusts. Hence, post-harvest organic minerals application as insecticide compare to synthetic insecticides provides no chemical residue, affordability, and virtually more benefit to environment with end users. Among inert dusts used in stored-product protection, Zeolites are included the group contain natural silica similarly diatomaceous earth. Review of entomology and agricultural literatures present insecticide potential of Zeolites formulations recently studied on Sitophilus zeamais Motschulsk, Rhyzopertha dominica (F.), Sitophilus oryzae (L.), Tribolium castaneum (Herbst), Lasioderma serricorne (F.), Tribolium confusum Jacquelin du Val, Meligethes spp., Aedes aegypti (L.), Cimex lectularius Linnaeus progressively.